- Argue that, if the initial concentrations of A and B
are a significant fraction of 1mol/L and the initial
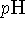 is not
too acidic, the slope of a
graph of
is not
too acidic, the slope of a
graph of 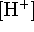 vs t will give the initial
rate for this reaction.
vs t will give the initial
rate for this reaction. - In one run, the following data were obtained:
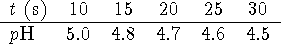
Calculate the initial rate.
![]()
- Determine the empirical rate law (order of reaction and rate constant).
- The structure of
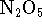 is
is
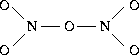 .
.
Do you think that this reaction is elementary? Briefly explain your reasoning.
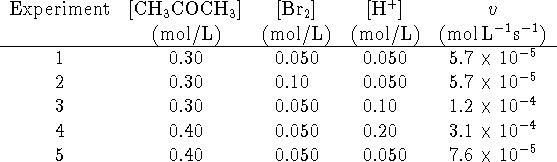
- Determine the rate law (orders and rate constant).
- Do you think that this reaction is elementary? Briefly explain your reasoning.
- Write down a complete set of rate equations for the
mechanism
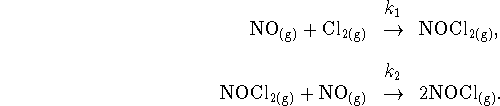
- What is the overall reaction?
- The empirical rate law for this reaction is
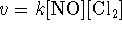 . What does this tell us
about the elementary reactions?
How is k related to the
rate constants of the two steps?
. What does this tell us
about the elementary reactions?
How is k related to the
rate constants of the two steps?
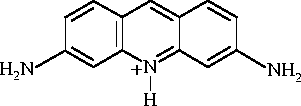
Like many other planar aromatic species, proflavin dimerizes in water:

where P is a proflavin ion and D is the dimer. The rate constants have been measured by a technique which we shall discuss later. They are
![]()
Calculate the equilibrium constant for the dimerization reaction.
- Obtain a differential equation involving only a for this reaction.
- Verify that
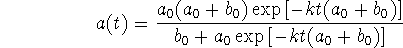
is a solution to your differential equation.
- The above solution can be rewritten in the form
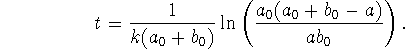
Obtain an expression for the half-life.
- Suppose that
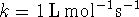 .
Compare the half-life when
.
Compare the half-life when 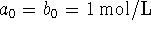 the its value when
the its value when
-
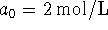 and
and 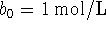 ;
; -
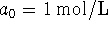 and
and 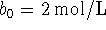 .
.
-
![]()
The reaction is either of the first or second order with respect to A and shows no dependence on the partial pressure of B. Determine the order and rate constant.
![]()
- Doubling times are computed on the assumption that the population is growing exponentially. What is the doubling time for the population of Finland?
- In what year would you predict that the population will reach six million?
- Is the population really growing exponentially?