- It sublimes.
- On the solid/liquid phase boundary, K=1. (Solid and liquid are in equilibrium and since the activities of pure solids and liquids are both 1, K=1.) In the portion of the phase diagram marked ``solid'', the solid, which is the reactant in the process described in this question, is favoured so we must have K<1.
- The liquid-vapour coexistence curve tells us the values of P and T at which water boils. We can see that this curve moves to larger values of T when P is raised. Therefore, the boiling temperature increases with pressure.
- Bonus:
- The triple occurs at a specific temperature.
Therefore, if we observe all three phases, we know
exactly what the temperature must be. This is different
from the behaviour of sublimation, melting and boiling
temperatures which all vary with pressure.
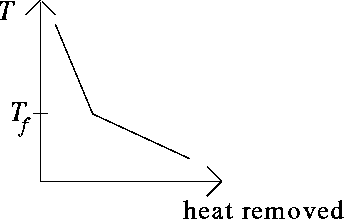
In the sketch, ![]() is the freezing temperature of the
solution. Since this is a solution, the solvent generally
freezes out first. Once freezing starts, removal of heat thus
causes some solvent to freeze, which leaves behind a more
concentrated solution whose temperature can thus drop a little
more.
is the freezing temperature of the
solution. Since this is a solution, the solvent generally
freezes out first. Once freezing starts, removal of heat thus
causes some solvent to freeze, which leaves behind a more
concentrated solution whose temperature can thus drop a little
more.
![]()
Since sodium fluoride dissociates in water, this represents 0.24mol of sodium ions and 0.24mol of fluoride ions.
For the water, we use the density to calculate the mass:
![]()
Using the molar mass, we then calculate the number of moles:
![]()
The mole fraction of water is therefore
![]()
Using Raoult's law, the vapour pressure is therefore
![]()
![]()
We convert this concentration to mol/L:
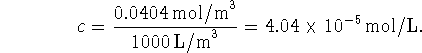
This means that the number of moles of protein in our flask should be about
![]()
If the molar mass of the protein is about 100000g/mol, we need
![]()
of protein.
- Bonus:
- Proteins are often not very soluble so it's often not
possible to make very concentrated solutions.
(There are several other good reasons for using low concentrations.)