Next: Useful information
Up: Back to the Chemistry 2000 test
index
Chemistry 2000, Section A
Spring 1996 Test 2
This exam will be marked out of 100.
The detailed breakdown for each question is given in each question.
The bonus questions are worth one mark each. Bonus marks
will be applied to your final grade, to a maximum of 15/15
for this section of the course (assignment 2 and this test).
- Here is a portion of the phase diagram of water:
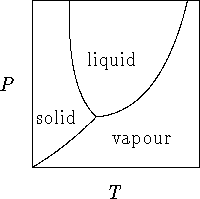
- What happens to ice
heated at a constant pressure
lower than the pressure at the triple point?
[10 marks]
- What can you say
about the size of the equilibrium
constant for the process
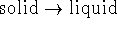 inside the solid region of the phase diagram?
[10 marks]
inside the solid region of the phase diagram?
[10 marks] - Does the boiling temperature
of water increase or decrease with
pressure? Explain how this information can be obtained from
the phase diagram.
[10 marks]
- Bonus:
- Why is the triple point useful for calibrating
thermometers?
- Explain what causes the appearance of dew
at night. [10 marks]
- Sketch the ideal cooling curve of a solution.
Clearly label the
temperature at which freezing starts. Explain the shape of your
curve. [20 marks]
- What is the vapour pressure of a solution
prepared by dissolving
10g of sodium fluoride in 100mL of water at
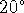 C?
The molar mass of sodium fluoride is 41.99g/mol. The vapour
pressure of pure water at
C?
The molar mass of sodium fluoride is 41.99g/mol. The vapour
pressure of pure water at 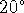 C is 2338Pa, its density is
0.9982g/mL and its molar mass is 18.02g/mol.
[20 marks]
C is 2338Pa, its density is
0.9982g/mL and its molar mass is 18.02g/mol.
[20 marks] - Suppose that you have an osmometer
designed to accurately measure
osmotic pressures between 50 and 3000Pa at
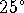 C.
You want to use it
to accurately
determine the molar mass of a protein whose molar mass you
have estimated by other means to be about 100000g/mol.
With a molecule this large, you should aim for the lower end of
the osmometer's range while leaving yourself a little room for
error so you decide on a target osmotic pressure of 100Pa.
You will use a 100mL volumetric flask to prepare the aqueous
protein solution.
How much of the protein should you
use for this determination?
[20 marks]
C.
You want to use it
to accurately
determine the molar mass of a protein whose molar mass you
have estimated by other means to be about 100000g/mol.
With a molecule this large, you should aim for the lower end of
the osmometer's range while leaving yourself a little room for
error so you decide on a target osmotic pressure of 100Pa.
You will use a 100mL volumetric flask to prepare the aqueous
protein solution.
How much of the protein should you
use for this determination?
[20 marks]
- Bonus:
- What are the practical reasons why should you aim
for the bottom of the osmometer's working range?
Next: Useful information
Up: Back to the Chemistry 2000 test
index
Marc Roussel
Tue Oct 15 15:46:27 MDT 1996

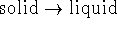 inside the solid region of the phase diagram?
[10 marks]
inside the solid region of the phase diagram?
[10 marks]