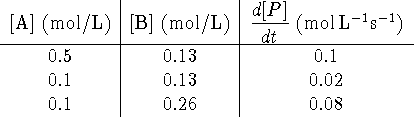
What is the experimental rate equation for [P]?

What is the order of the reaction with respect to A and what is the rate constant?
- Sulphur dioxide is formed from sulphur monoxide in the atmosphere
by the elementary reaction
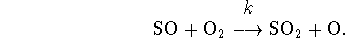
For this reaction,
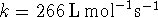 at
at
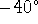 C and
C and
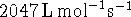 at
at
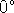 C. What is
the activation energy of the reaction?
C. What is
the activation energy of the reaction?
- Bonus:
- Also calculate
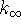 .
.
- Write down the rate equations for the mechanism

- Write down an expression for the rate of formation of the product P if the first step is rate-limiting.
- Bonus: What would the experimental rate law for the formation of P be if the first step were rate-limiting and the reactant A were present in huge excess?