![]()
The activities of the nickel and sulfide ions are related by
![]() . Therefore
. Therefore
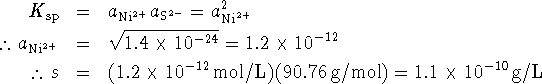
![]()
Thus we have . However, by stoichiometry, . We can find the activity of hydroxide from the pH:
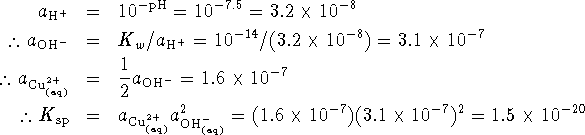
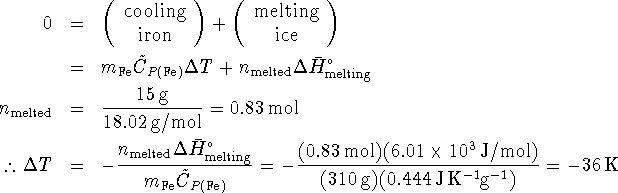
![]() .
.
![]() so the initial temperature
of the iron was
so the initial temperature
of the iron was ![]() or 309K.
or 309K.
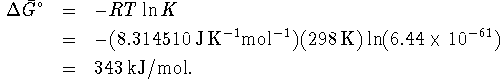
However, ![]() since the free
energies
of formation of boron and of hydrogen are both zero. Therefore
since the free
energies
of formation of boron and of hydrogen are both zero. Therefore
![]()
- This is calculated from the free energies of formations
of the reactants and products:
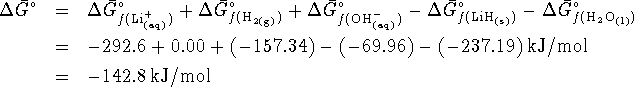
- To calculate
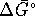 at any temperature
other than 298K, we need
at any temperature
other than 298K, we need 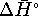 and
and 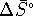 .
.
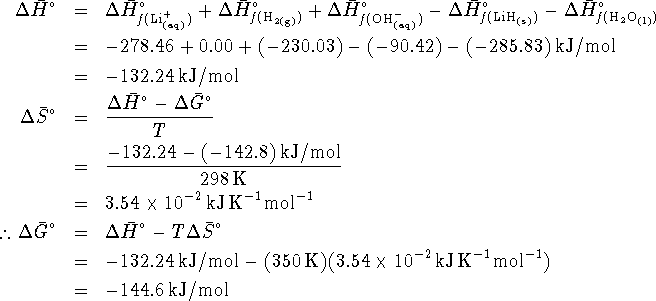
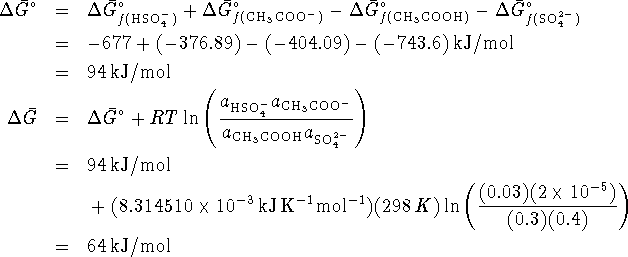
Since ![]() , the reaction is not spontaneous.
, the reaction is not spontaneous.