Next: Useful data
Up: Back to the Chemistry 2000 test
index
Chemistry 2000, Fall 96, Test 3
Answer any five questions.
- The solubility product of nickel (II) sulfide (NiS) at
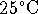 is
is
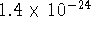 and its molar mass is 90.76g/mol. What
is the solubility of nickel sulfide in g/L?
and its molar mass is 90.76g/mol. What
is the solubility of nickel sulfide in g/L? - At 298K, the pH of a saturated solution of copper (II) hydroxide
(
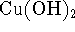 ) is 7.5. What is
) is 7.5. What is 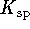 ?
? - A 310g piece of iron (specific heat capacity
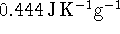 )
is packed in ice at
)
is packed in ice at 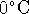 . When
equilibrium is reached (i.e. when the temperature of the iron
is equal to the temperature of the ice), 15g of ice have
melted. What was the initial temperature of the iron? The
heat of melting of ice is 6.01kJ/mol and the molar mass of
water is 18.02g/mol.
. When
equilibrium is reached (i.e. when the temperature of the iron
is equal to the temperature of the ice), 15g of ice have
melted. What was the initial temperature of the iron? The
heat of melting of ice is 6.01kJ/mol and the molar mass of
water is 18.02g/mol. - For the reaction
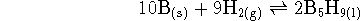
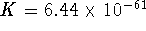 at 298K. What is the
standard free energy of formation of
at 298K. What is the
standard free energy of formation of 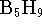 ?
?
-
- Calculate
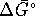 at 298K for the reaction
at 298K for the reaction
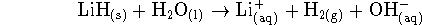
- Calculate
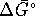 at 350K for the same
reaction.
at 350K for the same
reaction.
- In a solution containing 0.4mol/L of
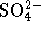 ,
0.3mol/L of
,
0.3mol/L of 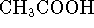 , 0.03mol/L of
, 0.03mol/L of
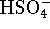 and
and 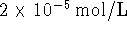 of
of 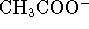 at
298K,
is the reaction
at
298K,
is the reaction
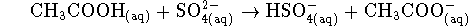
spontaneous?
Marc Roussel
Thu Nov 21 13:41:19 MST 1996
![]()
![]() at 298K. What is the
standard free energy of formation of
at 298K. What is the
standard free energy of formation of ![]() ?
?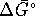 at 298K for the reaction
at 298K for the reaction
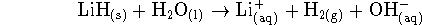
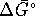 at 350K for the same
reaction.
at 350K for the same
reaction.
![]()
![]()
![]() at 298K. What is the
standard free energy of formation of
at 298K. What is the
standard free energy of formation of ![]() ?
?![]()
![]()