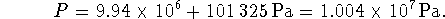- Solutes depress the freezing points of solvents. Since salt is very soluble in water, salt-water solutions can have very low freezing points.
- Ethanol is volatile. It might melt the ice on a road very quickly, but it would quickly evaporate and the ice would refreeze.
![]()
In water, the sodium sulfite will dissociate into 1.98mol of
![]() and 3.96mol of
and 3.96mol of ![]() ions.
Using the molar density of water, we have
ions.
Using the molar density of water, we have
![]()
The mole fraction of water is therefore
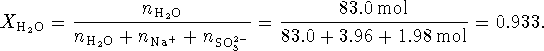
Since only the water is volatile in this solution, the vapour pressure of the solution is entirely due to the water:
![]()
-
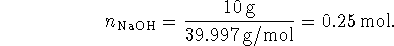
The concentration of hydroxide is therefore
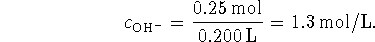
The activity of hydroxide is 1.3. From the
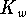 equation, we have
equation, we have
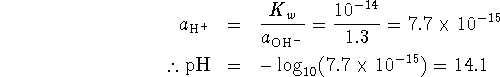
-
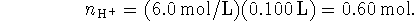
Some of the acid is neutralized by the base, so after the addition, we have
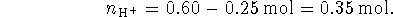
The total volume after the addition is 300mL so
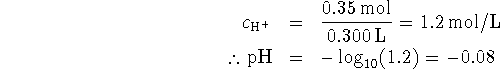
- None of the protons of phosphoric acid
have
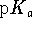 's reasonably close to 4.
The nearest is the first proton which has a
's reasonably close to 4.
The nearest is the first proton which has a
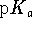 1.9 pH units removed from 4. Since
effective buffering requires similar amounts of the acid
and base form and since this is achieved when the pH is
similar to the
1.9 pH units removed from 4. Since
effective buffering requires similar amounts of the acid
and base form and since this is achieved when the pH is
similar to the 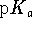 , acid phosphates are
probably not the right materials from which to make a pH
4 buffer.
, acid phosphates are
probably not the right materials from which to make a pH
4 buffer. - The
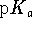 of the third proton is nearly 12
so a pH 12 buffer can be made from sodium hydrogen
phosphate and sodium phosphate.
of the third proton is nearly 12
so a pH 12 buffer can be made from sodium hydrogen
phosphate and sodium phosphate. - The buffer will use the last acid equilibrium of
the acid phosphates, namely
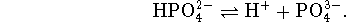
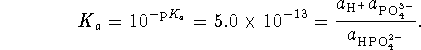
We want a pH 12 buffer so
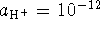 .
Therefore
.
Therefore
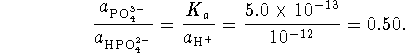
We also want
 .
Putting the two equations together, we get
.
Putting the two equations together, we get

We must dissolve 468g of sodium hydrogen phosphate and 279g of sodium phosphate in 10L of water.
- The reaction ratio is
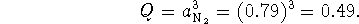
Since Q<K, the reaction will spontaneously occur as written, i.e. lead azide will be converted to lead and nitrogen. Furthermore, since this reaction can't materially affect the partial pressure of nitrogen in the atmosphere (the atmosphere is too big for that), the reaction will continue until the lead azide runs out. Thus, at equilibrium, only lead remains.
- Because the equilibrium constant is huge, the lead azide
is quantitatively converted to lead and nitrogen.
Initially
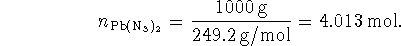
Since 3
 molecules are generated for every
formula unit of lead azide,
molecules are generated for every
formula unit of lead azide, 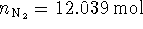 at equilibrium. The pressure due
to this added nitrogen is
at equilibrium. The pressure due
to this added nitrogen is
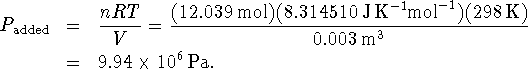
There was already 1atm = 101325Pa of nitrogen in the box so