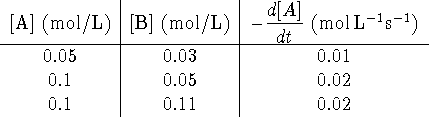
What is the experimental rate law? Also, calculate the rate constant. [10 marks]

Classify all of the species as reactant, product, intermediate or catalyst. [10 marks]
![]()
- Define half-life. [2 marks]
- Derive an expression for the half-life of a third-order reaction in terms of the initial concentration of the reactant and the rate constant. [8 marks]
- For [A] in mol/L and time in s, what are the units of a third-order rate constant? Hint: This is just a matter of unit analysis. [2 marks]
- You are told that a certain reaction obeys third-order kinetics. Explain how you would use this equation along with data giving [A] as a function of time to obtain the rate constant. [8 marks]