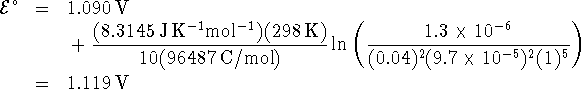![]()
and
![]()
The spontaneous overall reaction is the one with a positive value of
![]() . Since the
. Since the ![]() term of the Nernst equation is
generally a small correction, we guess
term of the Nernst equation is
generally a small correction, we guess
![]()
(If we have guessed wrong, we will get a negative voltage and we will know that the spontaneous reaction in fact runs in the opposite direction.) We compute the electrochemical potential from the Nernst equation:
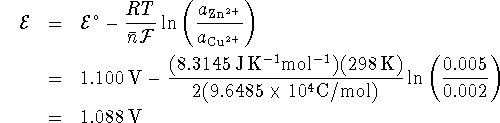
![]()
and
![]()
The overall reaction is therefore
![]()
with ![]() and
and ![]() .
From the Nernst equation, we have
.
From the Nernst equation, we have
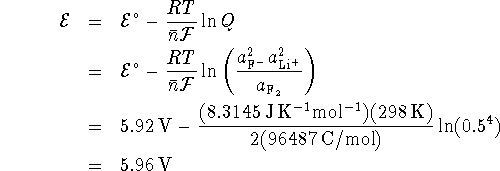
-
- One of the half-reactions is the standard
hydrogen electrode. Thus we only need to
balance the other half-reaction to start with.
The other half-reaction turns iodate ions into
iodine:

-

-
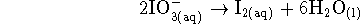
-
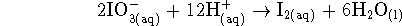
-
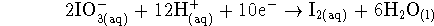
Now that we know that iodate is reduced, the other half-reaction must be the oxidation of hydrogen:
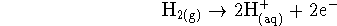
- If we multiply the second half-reaction by five
and add this to the first half-reaction, we get
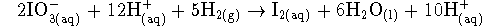
or, after simplification,
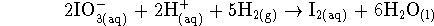
- One of the half-reactions is the standard
hydrogen electrode. Thus we only need to
balance the other half-reaction to start with.
The other half-reaction turns iodate ions into
iodine:
- We need the activities of all the reactants
and products. The activity of iodine is
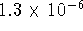 . We started out with 0.04mol/L of iodate
ions but each iodine formed requires the reaction of two
iodate ions so
. We started out with 0.04mol/L of iodate
ions but each iodine formed requires the reaction of two
iodate ions so 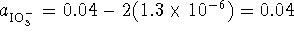 . The initial pH is 4 so we started out
with
. The initial pH is 4 so we started out
with 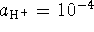 . Again, we need two
hydrogen ions per iodine so at the time of measurement,
. Again, we need two
hydrogen ions per iodine so at the time of measurement,
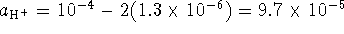 . The activity of hydrogen gas is 1.
Therefore, by rearrangement of the Nernst equation,
. The activity of hydrogen gas is 1.
Therefore, by rearrangement of the Nernst equation,
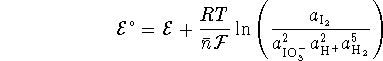
Referring back to the balancing process, we see that
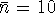 . Therefore
. Therefore