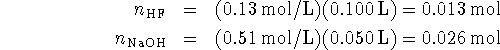
There is more than enough sodium hydroxide to neutralize the acid. The excess hydroxide is found by difference:
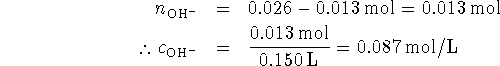
(Note that the total volume after mixing the two solutions is
150mL.)
To find the pH, we first calculate ![]() :
:
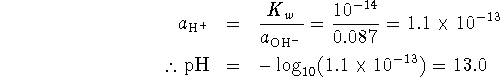
![]()
One barium ion is generated for each carbonate ion so
![]()
The solubility is therefore ![]() .
However we want our answer in g/L. The conversion is made with
the molar mass of barium carbonate:
.
However we want our answer in g/L. The conversion is made with
the molar mass of barium carbonate:
![]()
![]()
![]()
If 335kJ of heat is used to bring the water to its boiling point, the remaining heat (115kJ) goes into evaporating water. The latent heat of vaporization of water is 40.79kJ/mol. The number of moles of water vaporized is therefore
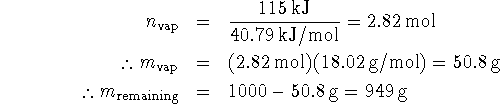
![]()
with an equilibrium constant
![]()
The sulfide is converted to ![]() as it is formed by
the equilibrium
as it is formed by
the equilibrium
![]()
for which
![]()
The overall reaction is therefore
![]()
The equilibrium constant for this reaction is
![]()
Since the pH of the buffer is 12, the activity of hydrogen ions
is ![]() . Furthermore, the stoichiometry of the reaction
results in one equivalent of
. Furthermore, the stoichiometry of the reaction
results in one equivalent of ![]() being formed for
each equivalent of
being formed for
each equivalent of ![]() :
:
![]()
Thus the solubility of lead sulfide in a pH 12 buffer is
![]() .
.