- The graphite-diamond coexistence curve slopes up as the temperature increases. Therefore, it is necessary to use higher pressures to get diamonds when the temperature is raised. It should be easier to make diamonds at lower temperatures.
- The diamond-liquid coexistence curve (i.e. the melting curve) has a positive slope. This means that as the pressure increases, the melting temperature increases.
- Since the diamond-graphite coexistence curve intersects
the P axis at
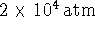 , 1atm
should be right at the bottom of this diagram.
The liquid phase is not observed at low pressures (lower
than the triple point which, from the diagram, occurs at
about
, 1atm
should be right at the bottom of this diagram.
The liquid phase is not observed at low pressures (lower
than the triple point which, from the diagram, occurs at
about 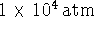 ). Therefore, if
graphite is heated above
). Therefore, if
graphite is heated above 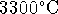 at
1atm, it should sublime.
at
1atm, it should sublime.
- The boiling temperature of water decreases as we decrease the pressure so we should be able to boil water by sufficiently reducing the pressure of gas over it.
- The temperature generally drops overnight. Vapour pressure decreases when the temperature is reduced. If the temperature drops enough, the partial pressure of water in the air becomes greater than the vapour pressure. The water vapour is no longer in equilibrium and it condenses. Condensation occurs preferentially on surfaces (like grass) acting as nucleators.
![]()
The pressures of hexane and cyclohexane in the mixture are related to their mole fractions so we have
![]()
Furthermore, the mole fractions of hexane and cyclohexane have to add to 1:
![]()
If we solve this last equation for ![]() and
substitute the result into the previous equation, we get
and
substitute the result into the previous equation, we get
![]()
This gives us ![]() and, therefore,
and, therefore,
![]() .
.
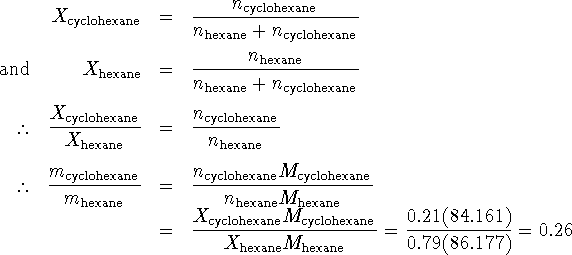
We want a percentage by mass so what we really want is
![]() .
If we divide the top and bottom of this expression by
.
If we divide the top and bottom of this expression by
![]() , we get
, we get
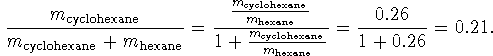
The mixture is 21% cyclohexane by mass.
![]()
Since NaCl dissociates into ![]() and
and ![]() ,
this is twice the concentration of either sodium or chloride
ions (i.e. twice the formal concentration of sodium chloride).
The formal concentration of sodium chloride should therefore be
1.4mol/kg.
We have 18000kg of solvent so
,
this is twice the concentration of either sodium or chloride
ions (i.e. twice the formal concentration of sodium chloride).
The formal concentration of sodium chloride should therefore be
1.4mol/kg.
We have 18000kg of solvent so
![]()
![]()
In the more conventional concentration units, this is
![]() . Since 100mL of water
(0.100L) of water was used, the number of moles of the
forensic sample is
. Since 100mL of water
(0.100L) of water was used, the number of moles of the
forensic sample is ![]() .
The mass was 2.841mg so the molar mass of the unknown
substance is
.
The mass was 2.841mg so the molar mass of the unknown
substance is
![]()
From the chemical formula, we know that the molar mass of MDA is 179.218g/mol. It seems quite likely that the material brought in by the police officer is MDA. Further tests on the sample should be ordered.