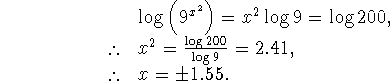
![]()
![]()
![]()
Would you believe that I didn't do it on purpose? The brain works in mysterious ways.
![]()
In the second solution,
![]()
The number of moles of chloride in the mixture is the sum of the amounts
in each solution so
![]() . We also need the number of moles of
water.
There is a total of 20mL of water. This corresponds to
. We also need the number of moles of
water.
There is a total of 20mL of water. This corresponds to
![]()
The mole fraction of the chloride ions is therefore
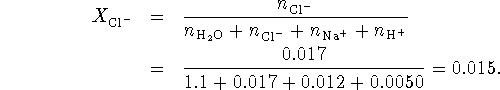
![]()
![]()
Multiply
this last number by two to get the number of sodium ions.
![]()
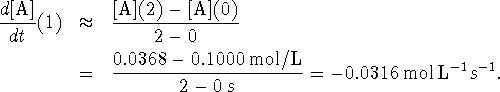
We do this for each point which is bracketed by two others to obtain the table
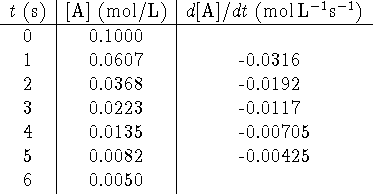
The graph has the following appearance:
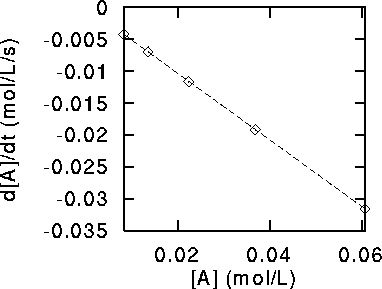
The points lie on a beautifully straight line. This is not a coincidence.