Chemistry 2000, Fall 96, Assignment 1
Due: September 12
All questions are equally weighted. Even when a question calls for a
yes or no answer, you should provide a detailed answer.
- Solve the equation
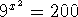 .
. - Solve the equation
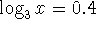 .
. - 10mL of a 1.2mol/L aqueous solution of sodium chloride is mixed
with 10mL of a 0.50mol/L aqueous solution of HCl. Take the
density of water in these solutions to be approximately
1.0g/mL and calculate the mole fraction
of chloride ions in the mixture.
- How many moles of sodium ions does 200g of a 0.035mol/kg
solution of sodium sulphate contain?
- Consider the following data obtained for a reaction in which A
is a reactant:
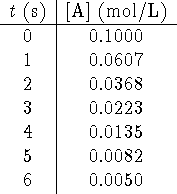
Plot 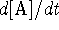 as a function of [A]. Do you recognize
the shape of the curve?
as a function of [A]. Do you recognize
the shape of the curve?
Marc Roussel
Mon Sep 2 14:53:43 MDT 1996

![]() as a function of [A]. Do you recognize
the shape of the curve?
as a function of [A]. Do you recognize
the shape of the curve?

![]() as a function of [A]. Do you recognize
the shape of the curve?
as a function of [A]. Do you recognize
the shape of the curve?