- n=2,
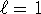 , m=-1
, m=-1 - The s label indicates
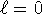 . The subscript means that m=1, but m
can't be larger than
. The subscript means that m=1, but m
can't be larger than 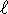 .
. - For n=3, there can't be f (
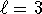 ) states.
) states.
![]()
For n=1 (the ground state), we get
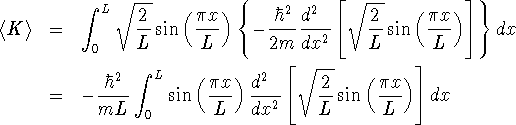
You could take the derivatives by hand (or get Maple to work
them out), but it's probably easier to go straight to Maple with
this expression:
> -hbar^2/(m*L)*int(sin(Pi*x/L) > *diff(sin(Pi*x/L),x$2),x=0..L);
![]()
Similarly, for n=2 (the first excited state)
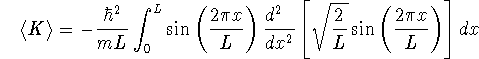
> -hbar^2/(m*L)*int(sin(2*Pi*x/L) > *diff(sin(2*Pi*x/L),x$2),x=0..L);
![]()
There was another way to do this problem. For the particle in a box,
![]() . Thus
. Thus ![]() (using the facts that
(using the facts that ![]() and that the
wavefunctions are normalized). You can verify that the answers given
above are exactly the energies of the ground and first excited states of
the particle in a box.
and that the
wavefunctions are normalized). You can verify that the answers given
above are exactly the energies of the ground and first excited states of
the particle in a box.
- Compatible observables can simultaneously take on precise values. Measurements of compatible observables need not disturb each other.
- To decide this, we need to work out the commutator of
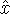 with
with 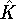 :
:
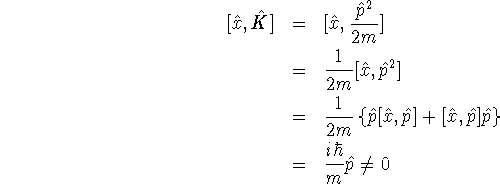
Since the commutator is not zero, the position and kinetic energy are not compatible.
- Using first-order perturbation theory, we have
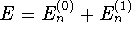 where
where 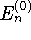 is the
particle-in-a-box energy and
is the
particle-in-a-box energy and 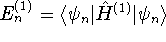 . In our
case,
. In our
case, 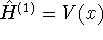 . Thus, for the ground
state,
. Thus, for the ground
state,
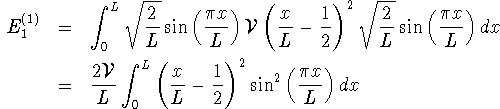
I called this quantity deltaE1 in my Maple session:
> deltaE1:=2*V/L*int(sin(Pi*x/L)^2 > *(x/L-1/2)^2,x=0..L);

Thus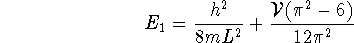
Similarly, for the first excited state:
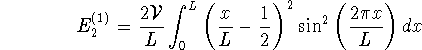
> deltaE2:=2*V/L*int(sin(2*Pi*x/L)^2 > *(x/L-1/2)^2,x=0..L);
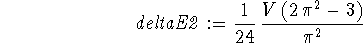
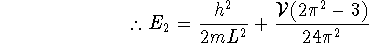
-

- To decide this, I first evaluated the numeric constant accompanying
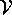 in deltaE1 and deltaE2:
in deltaE1 and deltaE2:
> evalf(deltaE1);
> evalf(deltaE2);
deltaE2 (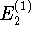 ) is bigger. To decide why that is, I
sketched the potential:
) is bigger. To decide why that is, I
sketched the potential:
> plot((x-1/2)^2,x=0..1);
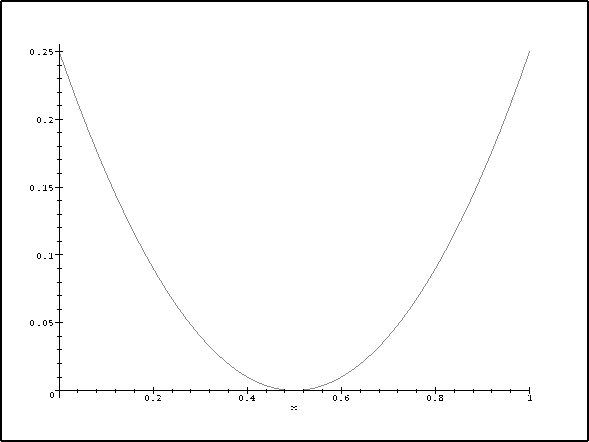
The energy of the n=2 level is most affected because the perturbation is greatest at the ends of the box and the electron density is greater at the ends for the n=2 state than for n=1 (which has its maximum density in the centre).
![]()
where ![]() is whatever region of space we are
interested in.
For the 1s state,
is whatever region of space we are
interested in.
For the 1s state,
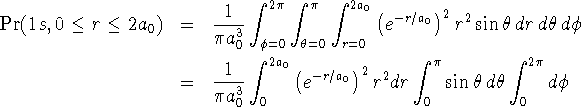
(I find it a little easier to keep track of all the brackets if
I break it up into a product of three integrals than if I try to
embed the integrals one into the other.)
> 1/(Pi*a0^3)*int(exp(-r/a0)^2*r^2,r=0..2*a0) > *int(sin(theta),theta=0..Pi) > *int(1,phi=0..2*Pi);
![]()
> simplify(");
![]()
> evalf(");
![]()
For the 2s state:
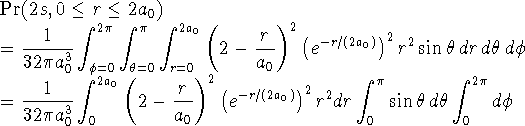
> 1/(32*Pi*a0^3) > *int((2-r/a0)^2*exp(-r/(2*a0))^2*r^2,r=0..2*a0) > *int(sin(theta),theta=0..Pi) > *int(1,phi=0..2*Pi);
![]()
> simplify(");
![]()
> evalf(");
![]()
Finally, to the probabilities of finding the electron between ![]() and
and
![]() in the two states, we just need to change the limits of
integration:
in the two states, we just need to change the limits of
integration:
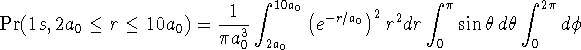
> 1/(Pi*a0^3)*int(exp(-r/a0)^2*r^2,r=2*a0..10*a0) > *int(sin(theta),theta=0..Pi) > *int(1,phi=0..2*Pi);
![]()
> simplify(");
![]()
> evalf(");
![]()
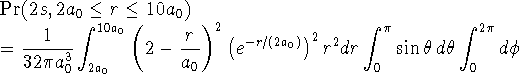
> 1/(32*Pi*a0^3) > *int((2-r/a0)^2*exp(-r/(2*a0))^2*r^2,r=2*a0..10*a0) > *int(sin(theta),theta=0..Pi) > *int(1,phi=0..2*Pi);
![]()
> simplify(");
![]()
> evalf(");
![]()