- Give the Hamiltonian and boundary conditions for the particle-in-a-box problem.
- Apply the variational method to the particle-in-a-box problem using a sine function as your trial wavefunction. Do you get all of the particle-in-a-box solutions by this method? Discuss.
![]()
where ![]() , R is the bond length, and
, R is the bond length, and ![]() is the equilibrium bond length.
is the equilibrium bond length.
- Write down the Born-Oppenheimer electronic Hamiltonian for LiH. If you use summation symbols in your Hamiltonian (a practice which I recommend), make sure to write down the summation limits explicitly.
- Explain how you would go about determining approximate formulas for the LiH molecular orbitals at a particular internuclear separation R.
- Explain why I specified ``at a particular internuclear separation'' in the previous question in relation to Born-Oppenheimer theory.
- Describe (in simple terms) the design of a Raman spectrometer. Explain (again, in simple terms) the operating principles involved.
- Name the frequency range in which the spectrometer should operate to study pure rotational transitions.
- Sketch the MO diagram necessary to treat second-row homonuclear diatomics. You may need to guess at the order of some of the MOs. The objective is to draw a reasonable MO diagram, not necessarily to get the ``correct'' MO diagram which must be determined experimentally.
- Use your MO diagram to predict the bond order of
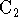 . Draw a Lewis diagram corresponding to
your predicted electronic configuration.
. Draw a Lewis diagram corresponding to
your predicted electronic configuration.
![]()
- Phenylalanine is a fluorescent amino acid. Its fluorescence lifetime in water is 6.4ns and the quantum yield for fluorescence is 0.004. Calculate the rate constants for fluorescence and thermal deactivation.
- In your opinion, would phenylalanine be a good pigment for fluorescent paints? Why or why not?