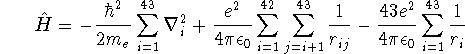
![]()
![]()
> psi2 := x -> (2*alpha/Pi)^(1/4)*(4*alpha*x^2-1)*exp(-alpha*x^2);
![]()
> V := x -> 1/2*k*x^2;
![]()
> assume(alpha>0); > int(psi2(x)^2*V(x),x=-infinity..infinity);
![]()
If we substitute the value of ![]() into this expression and
simplify, we get
into this expression and
simplify, we get
![]() . This is exactly half the
energy of this state.
. This is exactly half the
energy of this state.
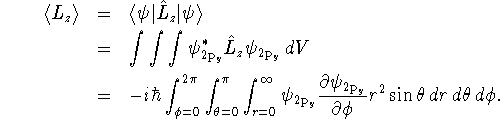
> psi2py := (r,theta,phi) -> > 1/sqrt(32*Pi*a0^5)*r*exp(-r/(2*a0))*sin(theta)*sin(phi);
![]()
> assume(a0>0); > -I*hbar*int(int(int(psi2py(r,theta,phi) > *diff(psi2py(r,theta,phi),phi)*r^2*sin(theta),theta=0..Pi), > phi=0..2*Pi),r=0..infinity);
![]()
Bonus: We obtain real-valued wavefunctions by adding
or subtracting wavefunctions with equal and opposite values of
![]() . The resulting wavefunctions are of the form
. The resulting wavefunctions are of the form
![]()
where N is a normalization constant ( ![]() ) and the
original wavefunctions are assumed to be normalized.
The average value of
) and the
original wavefunctions are assumed to be normalized.
The average value of ![]() is obtained by
is obtained by
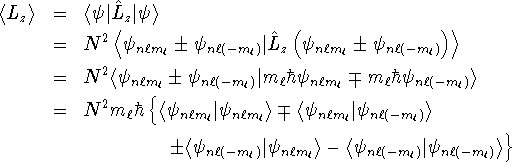
The inner products ![]() and
and ![]() are both zero because they are inner products of wavefunctions
which are eigenfunctions of a Hermitian operator (
are both zero because they are inner products of wavefunctions
which are eigenfunctions of a Hermitian operator ( ![]() )
corresponding to different eigenvalues. The other two are
normalization integrals so they are both equal to 1. Therefore
)
corresponding to different eigenvalues. The other two are
normalization integrals so they are both equal to 1. Therefore
![]() .
.
> phi := x -> sin(Pi*x/L) + a*sin(Pi*x/L)^2;
![]()
We compute the variational energy in pieces.
> Kip := -hbar^2/(2*m)*int(phi(x)*diff(phi(x),x$2),x=0..L);
![]()
> V := x -> k/2*(x-L/2)^2;
![]()
> Vip := int(phi(x)^2*V(x),x=0..L);
![]()
> ip := int(phi(x)^2,x=0..L);
![]()
Let us define the parameters before proceeding any further:
> k:=0.002;
![]()
> m:=1e-26;
![]()
> L:=0.3e-9;
![]()
> h := 6.6261e-34;
![]()
> hbar := h/(2*Pi);
![]()
We can now compute the variational energy:
> Evar := (Kip+Vip)/ip;
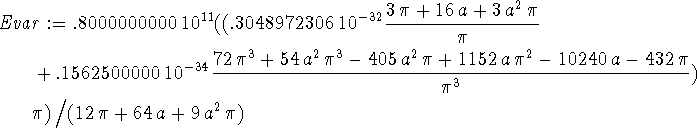
> sa:=solve(diff(Evar,a)=0,a);
![]()
> Evar1 := evalf(subs(a=sa[1],Evar));
![]()
> Evar2 := evalf(subs(a=sa[2],Evar));
![]()
Evar2 is the lower energy so it is our approximation to the ground-state energy. Note that the corresponding value of a is small and that the first part of the variational wavefunction is just a particle-in-a-box wavefunction. This means that the solution is very much like that of the particle-in-a-box problem and, by implication, less like the solution of the harmonic oscillator.