


Up: Back to the Chemistry 3730 test
index
Chemistry 3730 Fall 1998 Test 1
Aids allowed: Maple, calculator
Useful information is provided at the back of this exam paper.
You can use Maple to solve the problems, but you must write down your
complete solution in the booklet provided. It is not necessary to write
down what you typed into Maple, but you should explain what you calculated
using normal mathematical notation.
- The spectrum of mercury has a bright blue line at 436nm.
Convert this wavelength to a frequency and to a wavenumber.
[2 marks]
- What is the minimum value of the square of the angular momentum
consistent with a z component of
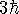 ?
[2 marks]
?
[2 marks] - Write down the Hamiltonian for a system consisting of
two electrons in a one-dimensional box of length L.
Explicitly state the boundary conditions of this problem.
[6 marks]
- Calculate the probability that a particle in a one-dimensional
box occupies the interval
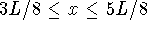 (the middle
quarter of the box) for n=1 and 2. Report your answers to
four decimal places.
Explain briefly why one answer is significantly larger than the
other. (Hint: What do the wavefunctions look like?)
[10 marks]
(the middle
quarter of the box) for n=1 and 2. Report your answers to
four decimal places.
Explain briefly why one answer is significantly larger than the
other. (Hint: What do the wavefunctions look like?)
[10 marks]
Maple hint:  is typed Pi in Maple.
is typed Pi in Maple.
- Calculate the following quantities for a particle in a
one-dimensional box in the n=1 energy level:
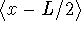 ,
,
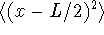 and
and
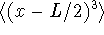 .
. Give exact answers.
[10 marks]
Give exact answers.
[10 marks]
Maple hints:  is typed Pi in Maple.
Be careful with your parentheses.
is typed Pi in Maple.
Be careful with your parentheses.
- Suppose that a proton is placed in a narrow box of
length 1cm at
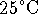 . The kinetic energy of a
particle confined in one dimension is approximately
. The kinetic energy of a
particle confined in one dimension is approximately
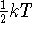 , where k is Boltzmann's constant, or
, where k is Boltzmann's constant, or
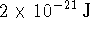 at
at 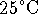 . Compute the
quantum number n of a proton in a box at this kinetic
energy. Then calculate the energy necessary for this particle to
make a transition from your computed value of n to n+1. Do
you expect quantum mechanical effects to be important in this
system?
The mass of a proton is
. Compute the
quantum number n of a proton in a box at this kinetic
energy. Then calculate the energy necessary for this particle to
make a transition from your computed value of n to n+1. Do
you expect quantum mechanical effects to be important in this
system?
The mass of a proton is 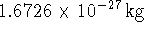 .
[10 marks]
.
[10 marks]
Hints: To calculate the transition energy, it will be convenient
to derive a formula first, and only then to plug in the numbers.
This is easy to do by hand, but if you want to use Maple to do
this, use simplify() to get the result in the simplest
possible form.
Quantum mechanical effects are important if the
separation of the energy levels is a large fraction of the energy
of the particle.
- We will see later that it is possible to use approximate
wavefunctions when we cannot solve a quantum mechanical problem
exactly. Suppose that you wanted to use

as an approximate wavefunction for a particle in a
one-dimensional box of length L. Derive a value for the normalization
constant A. [10 marks]
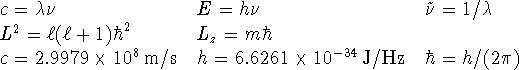
Electrostatic potential energy:
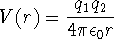
For a particle in a one-dimensional box of length L,
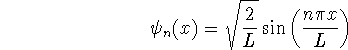
and
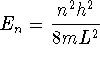 .
.



Up: Back to the Chemistry 3730 test
index
Marc Roussel
Mon Oct 5 14:39:13 MDT 1998
![]() is typed Pi in Maple.
is typed Pi in Maple.![]() is typed Pi in Maple.
Be careful with your parentheses.
is typed Pi in Maple.
Be careful with your parentheses.![]()
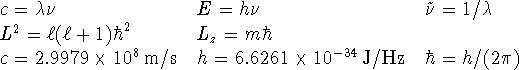
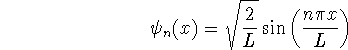
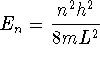 .
.