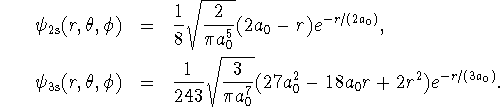
Hint: No integration is required to solve this problem.
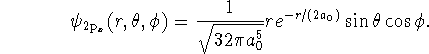
Hints: Maple will need to know that ![]() . The volume element
in spherical polar coordinates is
. The volume element
in spherical polar coordinates is ![]() . The variables run over the
following ranges:
. The variables run over the
following ranges:
![]() ,
, ![]() ,
, ![]() .
.
![]()
where ![]() .
Normalize this wavefunction. Report your normalization factor
in terms of
.
Normalize this wavefunction. Report your normalization factor
in terms of ![]() .
.
Hint: Don't define ![]() in your Maple session but do tell
Maple that this parameter is positive.
in your Maple session but do tell
Maple that this parameter is positive.
![]()
What is the percent error in the ground-state energy using this trial wavefunction?
Reminder: The particle-in-a-box ground-state energy is
![]() .
.