![]()
As written, the wavefunction is not normalized.
- Normalize this wavefunction.
- Calculate the average kinetic energy.
- Plot the radial probability density.
- Calculate the probability that
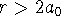 for a hydrogen
atom in this state. Give a floating-point answer with at least four significant
figures.
for a hydrogen
atom in this state. Give a floating-point answer with at least four significant
figures.
Hints: Maple will need to know that ![]() . The volume element
in spherical polar coordinates is
. The volume element
in spherical polar coordinates is
![]()
The angles ![]() and
and ![]() run over the following ranges:
run over the following ranges:
![]() ,
, ![]() . The Laplacian operator
is spherical polar coordinates is
. The Laplacian operator
is spherical polar coordinates is
![]()
To plot the radial probability density, use the following Maple
command:
plot(subs(a0=1,rpd),r=0..?);
where rpd is your radial probability density and ?
is replaced by some suitable number.
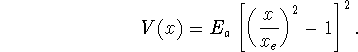
- Plot the potential energy.
Note the symmetric double well shape with an intervening energy
barrier.
This might for instance be the potential energy profile for a
proton transfer between two chemically similar group. Such
reactions are common in the active sites of enzymes.
Maple hint: To scale the x axis in units of
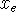 and
the energy axis in units of
and
the energy axis in units of 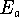 , use the following
plot command:
, use the following
plot command:
plot(subs(xe=1,Ea=1,V(x)),x=-?..?);where?is replaced by a suitable number. - Write down the Hamiltonian for a proton subjected to this potential energy.
- The probability density should have maxima corresponding
to the two potential energy wells. A reasonable guess
for the wavefunction
would be a superposition of Gaussian functions with
their maxima at
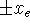 .
Therefore use the following variational wavefunction to
find an approximation to the ground state wavefunction:
.
Therefore use the following variational wavefunction to
find an approximation to the ground state wavefunction:
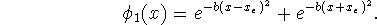
Maple hints: b>0. Maple's answer will be a complicated expression involving the
RootOf()operator. Save this expression in a variable, i.e. type something likebest_b := solve(...);. - Suppose that
we are dealing with a proton transfer reaction for which
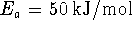 and
and 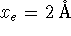 .
The mass of a proton is
.
The mass of a proton is
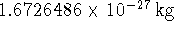 .
Define these values in your Maple worksheet, along with
.
Define these values in your Maple worksheet, along with
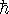 , and use
, and use
evalf()to find the best value of b. Don't assign this value to b. Instead, usesubs()to substitute it into your variational energy, andevalf()to get a floating-point value. Store this value in a variable with a distinctive name (say, E0). - Find the points where
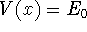 , where
, where 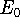 is your approximation to the ground state energy.
Then calculate the probability that the proton is to be
found in the activation barrier between the two wells.
In your opinion, is tunneling an important process for
this system?
is your approximation to the ground state energy.
Then calculate the probability that the proton is to be
found in the activation barrier between the two wells.
In your opinion, is tunneling an important process for
this system?
Maple hint: You will have to use
subs()to substitute b into your wavefunction.