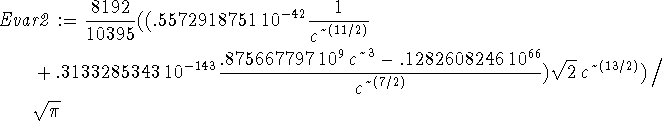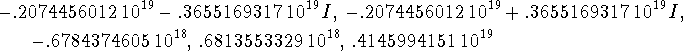The potential energy is
> V := r -> epsilon*((sigma/r)^12-(sigma/r)^6);
![]()
> epsilon := 1.71e-21;
![]()
> sigma := 342e-12;
![]()
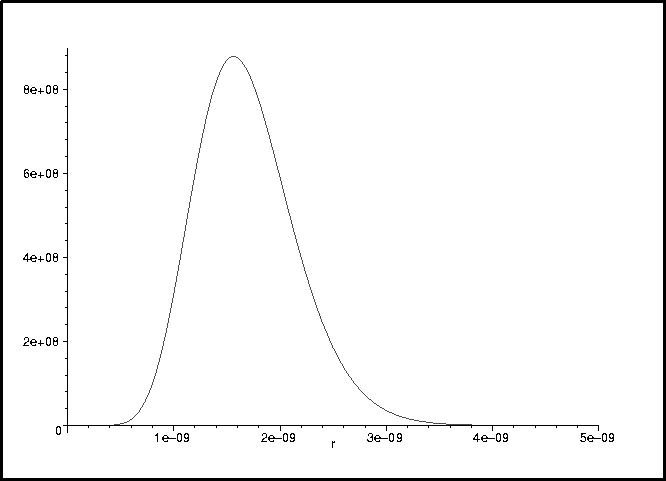
> plot(V(r),r=0..1e-9,-1e-21..1e-21);
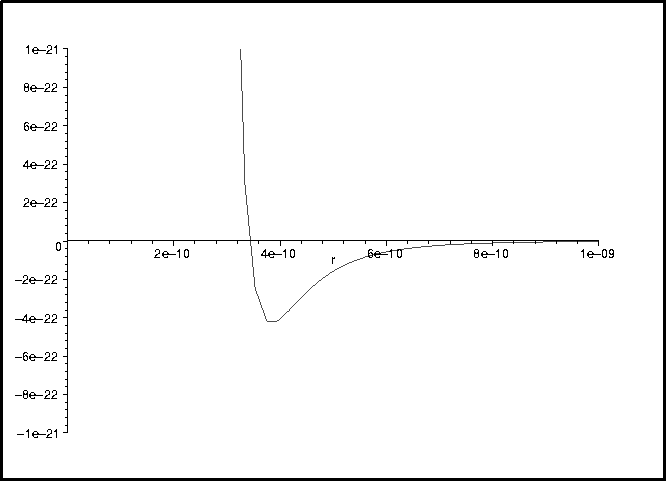
![]()
The mass of one argon atom, in kg, is
> mAr := 39.962384/1000/6.02214e23;
![]()
The reduced mass of two argon atoms is therefore
> mu := 1/(1/mAr+1/mAr);
![]()
We also need ![]() :
:
> hbar := 6.62608e-34/(2*Pi);
![]()
> phi := r -> r^6*exp(-b*r);
![]()
> assume(b>0);The variational energy is
> Kip := int(phi(r)*(-hbar^2/(2*mu))*diff(phi(r),r$2),r=0..infinity);
![]()
> Vip := int(phi(r)*V(r)*phi(r),r=0..infinity);
![]()
> ip := int(phi(r)^2,r=0..infinity);
![]()
> Evar:=(Kip+Vip)/ip;
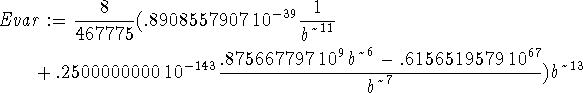
The best wavefunction minimizes the variational energy. In this
calculation, the variational parameter is b so we want to minimize
![]() with respect to b.
with respect to b.
> solve(diff(Evar,b)=0,b);

We want a real, positive b. (It is possible for b to be complex with a positive real part. However, if you try substituting the complex solutions into the variational energy, you will find that in this case they all give complex energies. Complex energies are nonsense so these complex roots are all spurious.) There are only two roots which might possibly be right. The best one gives the lowest energy.
> subs(b=2109144137.,Evar);
![]()
> subs(b=3839407642.,Evar);
![]()
The second solution gives the lower energy so it is the right one. Note in fact that the first value of b tried gives a positive energy. This is the equivalent, for intermolecular forces, of an antibonding state.
> b := 3839407642;
![]()
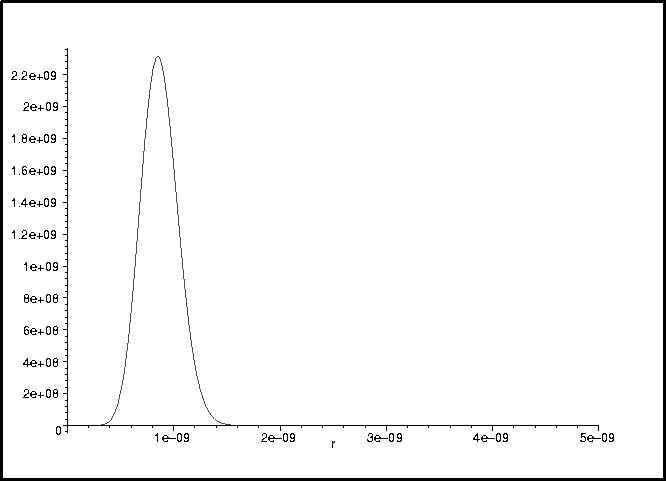
The normalized wavefunction is calculated by
> ip := int(phi(r)^2,r=0..infinity);
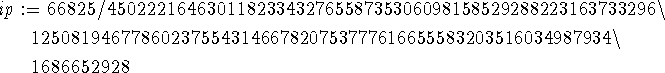
> phi_normalized := r -> phi(r)/sqrt(ip);
![]()
Verification:
> int(phi_normalized(r)^2,r=0..infinity);
![]()
> plot(phi_normalized(r)^2,r=0..5e-9);