Due: 10:00 a.m., Wednesday, Dec. 2
In this assignment, we will be studying the effective potential energy curves
of ![]() in the simple LCAO approximation studied in class.
You will recall that the electronic energies of the two states are given
by
in the simple LCAO approximation studied in class.
You will recall that the electronic energies of the two states are given
by
![]()
where ![]() and
and ![]() . These integrals can be
evaluated analytically in a special coordinate system in terms of the
internuclear distance R:
. These integrals can be
evaluated analytically in a special coordinate system in terms of the
internuclear distance R:
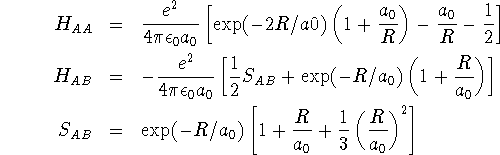
Maple hint: Maple will need to know that ![]() is a positive
number before you attempt to take limits.
is a positive
number before you attempt to take limits.
![]()
for each of the two states. Enter values into your Maple worksheet for the constants, as follows:
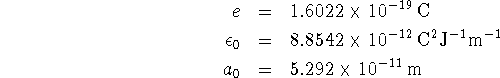
Plot both effective potential energy curves on the same set of axes. Observe that one of them is consistently lower in energy and has a minimum. This is the ground (bonding) MO. The other is the antibonding MO.
Hints: Let's say you called your two effective potential
energy curves Veffp(R) and Veffm(R). To plot
them on the same axes, use the following Maple command:
plot({Veffp,Veffm},0..?,??..???);
where ?, ?? and ??? are replaced by
suitable plotting limits.
To figure out which color corresponds to which curve, evaluate
the effective potential energies at a particular value of R.
Maple hint: Use the following Maple command to solve the
equation:
fsolve(eq,R,?..??);
where eq is your equation and ? and ??
are two values of R which bracket the minimum. (Read these
off your graph.)
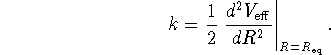
From the force constant, calculate the approximate zero-point
vibrational energy ![]() . Use
the following data:
the mass of a hydrogen nucleus is 1.0078amu,
. Use
the following data:
the mass of a hydrogen nucleus is 1.0078amu, ![]() and
and ![]() .
.
Maple hints: Use subs() to substitute your equilibrium bond length into k and use evalf() to force evaluation.