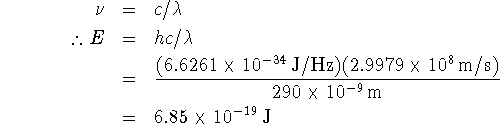
![]()
Thus, for this experiment, it is claimed that the product
![]() can be as small as
can be as small as ![]() . On the other hand,
. On the other hand, ![]() . The
claimed accuracy of the experiment is much greater than is
permitted by the uncertainty principle. The author of the
article has either made a mistake or has found a way around a
fundamental limitation of quantum mechanics. The former is
considerably more likely.
. The
claimed accuracy of the experiment is much greater than is
permitted by the uncertainty principle. The author of the
article has either made a mistake or has found a way around a
fundamental limitation of quantum mechanics. The former is
considerably more likely.
> psi := (n,L,x) -> sqrt(2/L)*sin(n*Pi*x/L);
![]()
The ground state of a particle in a box is the n=1 state.
The probability is
> int(psi(1,L,x)^2,x=3*L/5..2*L/3);
![]()
> evalf(");
![]()
> int(psi(3,L,x)*x^15*psi(3,L,x),x=0..L);
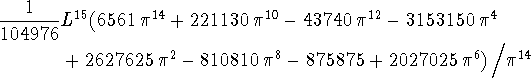
> evalf(");
![]()
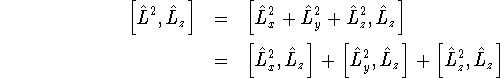
The last term is zero because after applying the commutator
simplification rules, it can only produce terms involving
![]() . We apply the commutator
rule for products to the other two terms:
. We apply the commutator
rule for products to the other two terms:
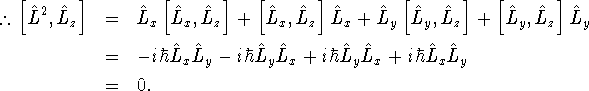
If the commutator of two observables is zero, they can be measured simultaneously without one measurement interfering with the other. We say that two such observables are compatible.
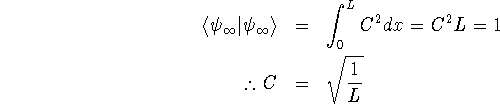
Normalization is required for
![]() to be interpretable as a probability
density.
to be interpretable as a probability
density.
- For n=1,
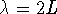 or
or 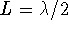 . For n=2,
. For n=2, 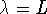 . For
n=3,
. For
n=3, 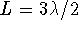 . The general pattern is
. The general pattern is
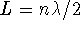 (an integer number of half-wavelengths
fits in the box) or, to put it the other way around,
(an integer number of half-wavelengths
fits in the box) or, to put it the other way around,

-
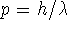

- For a particle in a box, all of the energy is kinetic and
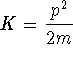 ,
,

Unfortunately, this kind of reasoning only works for a very small number of quantum mechanical problems.