


Next: Useful information
Up: Back to the Chemistry 3730 test
index
Chemistry 3730 Fall 1997 Test 1
Duration: 90 minutes
Aids permitted: calculator, Maple
All answers must be recorded in full in the exam booklets provided. If
you use Maple, you should explain what you used it for, although you
need not write down every Maple command which you used to solve a
problem.
The value of each question is noted. The aggregate value of all
questions is 50.
Note the useful information which follows the questions.
Answer all questions.
- The ultraviolet spectrum of a molecule has a band centered
at a wavelength of 290nm. What is the energy of the
photons associated with this transition?
[3 marks]
- Suppose that you are assigned to referee a journal article
for the prestigious Physical Review. The
author of the article claims to have designed an experiment
which measures proton positions to an accuracy of
1Å (
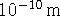 )
while simultaneously measuring velocities between 1 and 1000m/s
to an accuracy of approximately
1%. Is this possible? The mass of a proton is
)
while simultaneously measuring velocities between 1 and 1000m/s
to an accuracy of approximately
1%. Is this possible? The mass of a proton is 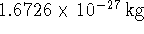 . [7 marks]
. [7 marks] - What is the probability that a particle in a box occupies the
interval
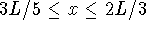 in the ground state?
Report your answer to four
significant figures. [8 marks]
in the ground state?
Report your answer to four
significant figures. [8 marks] - Calculate the expectation value of
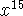 for a particle in
a box in the n=3 state. Report your answer to four
significant figures. [10 marks]
for a particle in
a box in the n=3 state. Report your answer to four
significant figures. [10 marks]
Note: Your answer may also contain some symbols.
- Using the relations

show that 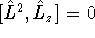 .
What is the physical interpretation of this result? [10 marks]
.
What is the physical interpretation of this result? [10 marks]
- In the limit as
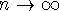 (the classical limit), the
wavefunction for a particle in a one-dimensional box
of length L
approaches
(the classical limit), the
wavefunction for a particle in a one-dimensional box
of length L
approaches
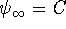 for some constant C. Find the value of C
which normalizes this wavefunction. What is the physical
significance of normalization? [6 marks]
for some constant C. Find the value of C
which normalizes this wavefunction. What is the physical
significance of normalization? [6 marks] - The particle-in-a-box energy levels can be derived using
simple arguments. The following steps will guide you through a
rederivation of this equation:
- Find a relationship between the wavelength of the
particle-in-a-box wavefunction and the length of the
box. [2 marks]
Hint: Sketch the wavefunction for a few values of n.
- Use de Broglie's relation to obtain the momentum.
[2 marks]
- Use the relationship between kinetic energy and momentum
to obtain an equation for the energy. [2 marks]



Next: Useful information
Up: Back to the Chemistry 3730 test
index
Marc Roussel
Mon Oct 6 15:38:51 MDT 1997

![]() .
What is the physical interpretation of this result? [10 marks]
.
What is the physical interpretation of this result? [10 marks]