- How many valence electrons would lithium have in this alternate Universe?
- Name the next element in lithium's group in this alternate Universe.
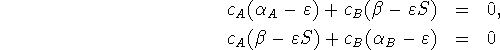
where ![]() and
and ![]() are the LCAO coefficients,
are the LCAO coefficients, ![]() ,
,
![]() and
and ![]() are integrals involving the
self-consistent field orbital Hamiltonian, S is the
overlap integral, and
are integrals involving the
self-consistent field orbital Hamiltonian, S is the
overlap integral, and ![]() is the orbital energy.
Find the orbital energy
is the orbital energy.
Find the orbital energy ![]() . If there is more than
one solution, explain how you would choose the best one given
the values of
. If there is more than
one solution, explain how you would choose the best one given
the values of ![]() ,
, ![]() ,
, ![]() and S.
and S.