- At a given magnetic field strength, how many energetically different spin states are there?
- In NMR, there is a selection rule: Only transitions
for which
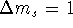 are observed. In the absence
of spin-spin coupling, how many lines would the
NMR spectrum of beryllium have?
are observed. In the absence
of spin-spin coupling, how many lines would the
NMR spectrum of beryllium have?
- Suppose that electrons had a spin of 3/2. How many main groups would there be?
- Describe the periodic table of a Universe where the electrons have a spin of 1. Hint: There is a qualitative difference between particles with integer and half-integer spin.
![]()
Determine the best value of the variational parameter ![]() and
the corresponding variational energy. Show that your result
conforms to the variational theorem. Normalize the
wavefunction.
and
the corresponding variational energy. Show that your result
conforms to the variational theorem. Normalize the
wavefunction.