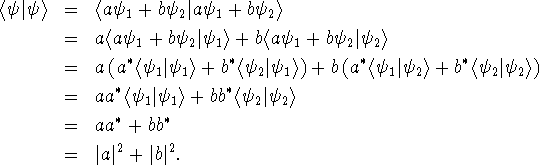
If the linear combination ![]() is normalized, we must
have
is normalized, we must
have ![]() .
.
![]()
If we plug in the numbers, we get ![]() . Of course, p = mv so
. Of course, p = mv so ![]() . For an electron, this gives
. For an electron, this gives ![]() . Needless to say, this is a very
large
error in the speed. For comparison, a free electron at room
temperature would have a speed of approximately
. Needless to say, this is a very
large
error in the speed. For comparison, a free electron at room
temperature would have a speed of approximately ![]() so the error imposed by a measurement of
position of the given accuracy is greater than typical
thermal velocities.
so the error imposed by a measurement of
position of the given accuracy is greater than typical
thermal velocities.
![]()
Schrödinger's equation is therefore
![]()
If the problem is separable, we should be able to write the
wavefunction in the form ![]() .
Making this substitution in Schrödinger's equation, we get
.
Making this substitution in Schrödinger's equation, we get
![]()
Divide both sides of the equation by ![]() and
rearrange:
and
rearrange:
![]()
The first parenthesis involves only terms which depend on x, the second only terms which depend on y. Since they add to a constant (E), it follows that each parenthesis must itself be a constant. We get two separate one-dimensional Schrödinger equations:
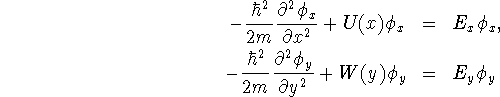
with ![]() .
.
- The particle-in-a-box energy equation is

The difference in energy between two adjacent energy levels is
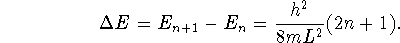
- Frequency is proportional to energy so the given frequency
is proportional to
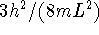 (using the above
formula with n=1). The next line would have an energy
proportional to
(using the above
formula with n=1). The next line would have an energy
proportional to 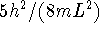 which is
which is 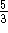 bigger than the energy of the first line, so the next
line in the spectrum should be found at
bigger than the energy of the first line, so the next
line in the spectrum should be found at 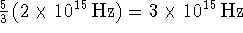 .
. - The energy of a photon is related to its frequency by
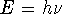 so in our case,
so in our case,
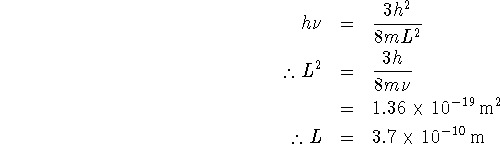
![]()
The two inner products are the same, so we are saying that the expectation value must be equal to its complex conjugate. The only way that can be true is if the imaginary part is zero, so the expectation value is real.