- Write down the full Hamiltonian of the diatomic molecule AlH. (Aluminium has atomic number 13.) [5 marks]
- Write down the electronic Hamiltonian of AlH. [3 marks]
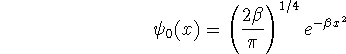
where
![]()
Calculate the average potential energy for a harmonic oscillator
in its ground state. Express your answer in multiples of
![]() [10 marks]
[10 marks]
Maple hints: Tell Maple that ![]() is positive but
don't otherwise define this parameter. Do the substitution by
hand in your test paper to complete the solution.
is positive but
don't otherwise define this parameter. Do the substitution by
hand in your test paper to complete the solution.
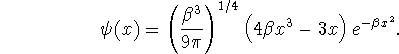
- Is this a solution of the quantum mechanical harmonic
oscillator problem? Explain in detail how you decided
this question. If it is a solution, give its quantum
number. [8 marks]
Maple hints: This time, define
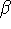 in terms of k,
in terms of k,
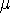 and
and 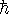 before you do any calculations.
before you do any calculations. - Is this wavefunction normalized? Again, explain
carefully how you decided this question. [4
marks]
Maple hints: Enter the following two commands into your Maple session before attempting this part:
> beta := 'beta'; > assume(beta>0);
These instructions undefine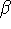 and instruct Maple
to treat it as a positive parameter.
and instruct Maple
to treat it as a positive parameter.
- Give an equation for the energy of a photon absorbed during a pure rotational transition of a rigid rotor. [3 marks]
- The lines of the rotational spectrum of AlH are spaced
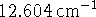 apart. What is the
equilibrium bond length? Aluminium has a single stable
isotope of mass
apart. What is the
equilibrium bond length? Aluminium has a single stable
isotope of mass 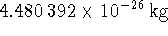 while the mass of
while the mass of 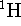 is
is 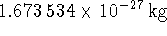 . [10 marks]
. [10 marks]
![]()
Using the variational wavefunction
![]()
find an approximation to the ground-state energy. [10 marks]
Maple hints: You will get more than one solution. Store them in
a variable called (say) sol. Then sol[1] is the
first solution, sol[2] is the second solution, and so on.
To substitute these values into another expression, use
subs(). For example, if you want to substitute a
variable called a by sol[1] into an expression
called b, you would type
> subs(a=sol[1],b);into your Maple session.
- ``Atoms'' of positronium (one electron and one positron, held together by the Coulomb force) can be produced and (quickly!) studied in high-energy accelerators. Positronium is of course a hydrogenic atom in which the ``nucleus'' and electron have the same mass. What is the ionization energy of positronium? [5 marks]
- What is the probability that the electron and positron
are within one Bohr radius (
 ) of each other in the
ground state of positronium? Give an exact algebraic
answer. [10 marks]
) of each other in the
ground state of positronium? Give an exact algebraic
answer. [10 marks]
Maple hint: You will have to tell Maple that
 is
positive.
is
positive. - Which is bigger, a hydrogen atom or a positronium atom? Explain how you arrived at your conclusion. [2 marks]
- The Born-Oppenheimer approximation
- Differences between quantum mechanics and classical mechanics
- Fourier Transform NMR
- The MO treatment of the hydrogen molecular ion
(
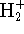 )
) - Refinements to the harmonic-oscillator, rigid-rotor model of a diatomic molecule
- The self-consistent field method
- Tunneling