> psi := (n,L,x) -> sqrt(2/L)*sin(n*Pi*x/L);
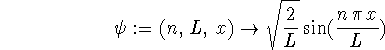
The perturbing potential is of the form
> V := x -> A*sin(2*Pi*x/Lambda);
![]()
We will need to tell Maple that n is an integer:
> assume(n,integer);
We now compute the energy shift caused by the perturbation:
> deltaE := int(psi(n,L,x)*V(x)*psi(n,L,x),x=0..L);
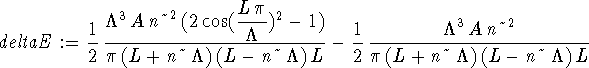
> simplify(deltaE);
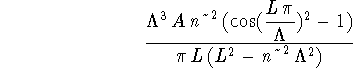
> psi1s := (r,theta,phi) -> sqrt(1/(Pi*a0^3))*exp(-r/a0);
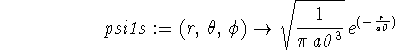
We will treat the ![]() term as a perturbation:
term as a perturbation:
V := r -> -e^2/(4*Pi*epsilon0)*k/r^2;
![]()
The energy correction for the 1s state is calculated by
> assume(a0>0); > deltaE:=int(int(int( psi1s(r,theta,phi)*V(r)*psi1s(r,theta,phi)*r^2*sin(theta), r=0..infinity),theta=0..Pi),phi=0..2*Pi);
![]()
The ionization energy is just the energy of the ground state (give or
take the sign).
The energy of the 1s state is ![]() .
This can be rewritten
in terms of
.
This can be rewritten
in terms of ![]() :
:
> E1s := -hbar^2/(2*a0^2*mu);
![]()
The ratio of the perturbation to the ground state energy is
> ratio := deltaE/E1s;
![]()
We want this ratio to be less than