


Up: Back to the Chemistry 3730 assignment
index
Chemistry 3730 assignment 5
Due: Friday, Oct. 10, 10:00 a.m.
- Calculate the probability that a particle in a box is located
between x=L/3 and x=L/2 for n=1 and n=2. Report your
answers as floating-point numbers. Explain the
difference in the probabilities.
- Show that the n=3 and n=212 states of the particle in a box
are orthogonal by an explicit calculation. Explain why the
calculation was a waste of time.
- Suppose that an electron is held in a one-dimensional box of
length L. An electric field produces a potential energy
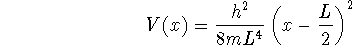
within the box. Compute an approximation to
the energies of the n=1 and n=2 levels of this system. In a
spectroscopic study, would a more or less energetic photon be
required to induce a transition between these levels than in the
absence of the electric field?
- For a particle in a square two-dimensional box of side L, the wavefunction
is of the form
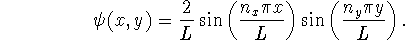
Note that the box is assumed to be in the conventional location,
i.e. in the positive quadrant with edges touching the x and
y axes.
- Calculate the probability that the particle is in the
square defined by
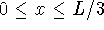 ,
, 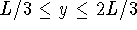 in the
in the 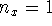 ,
, 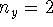 and
and 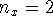 ,
, 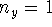 states.
Give your answer in floating-point form.
states.
Give your answer in floating-point form. - Calculate the average of
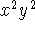 in the
in the 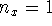 ,
, 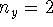 state.
state.
Marc Roussel
Fri Sep 26 11:40:43 MDT 1997
![]()
![]()
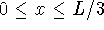 ,
, 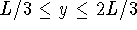 in the
in the 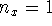 ,
, 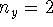 and
and 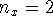 ,
, 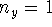 states.
Give your answer in floating-point form.
states.
Give your answer in floating-point form.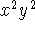 in the
in the 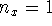 ,
, 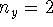 state.
state.