> psi := (n,L,x) -> sqrt(2/L)*sin(n*Pi*x/L);
![]()
> V := (L,x) -> Pi*h^2/(30*m*L^2)*sin(3*Pi*x/L);
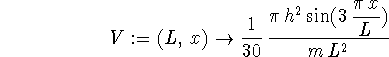
For n=1,
> deltaE1 := int(psi(1,L,x)*V(L,x)*psi(1,L,x),x=0..L);
![]()
For n=2,
> deltaE2 := int(psi(2,L,x)*V(L,x)*psi(2,L,x),x=0..L);
![]()
For n=3,
> deltaE3 := int(psi(3,L,x)*V(L,x)*psi(3,L,x),x=0..L);
![]()
> evalf(deltaE1);
![]()
> evalf(deltaE2);
![]()
> evalf(deltaE3);
![]()
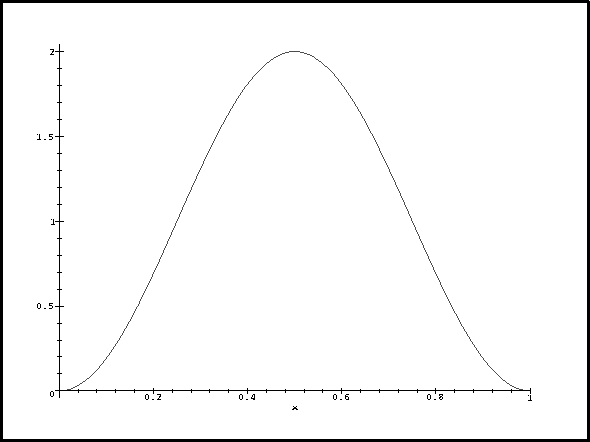
The energy correction is greatest for n=2. Let's look at the probability densities and perturbing potential. To plot the densities, we'll pick a box of length 1. The density for n=1 looks like
> plot(psi(1,1,x)^2,x=0..1);
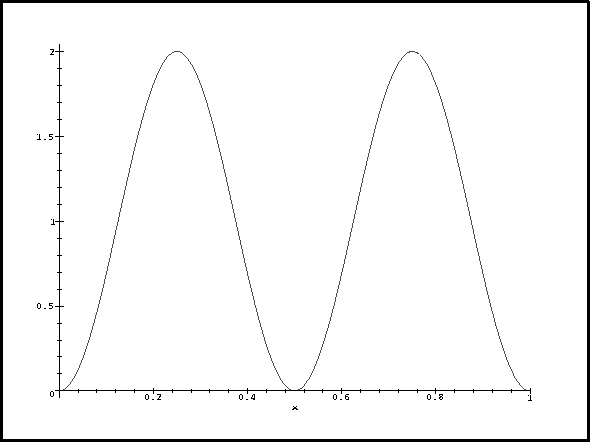
For n=2, we have
> plot(psi(2,1,x)^2,x=0..1);
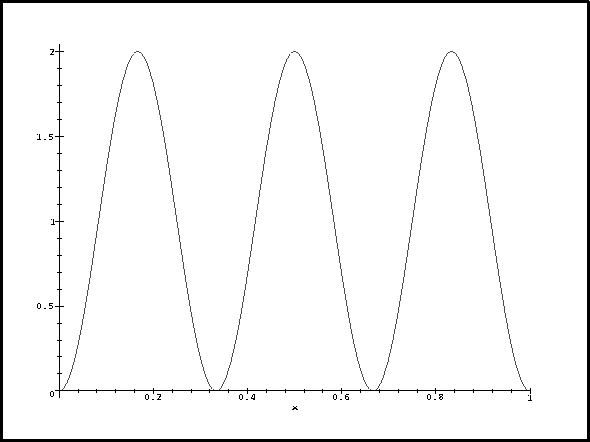
And, of course, for n=3,
> plot(psi(3,1,x)^2,x=0..1);
The perturbing potential involves h and m, neither
of which we really want to deal with directly, so let's plot
![]() :
:
> plot(V(1,x)*m/h^2,x=0..1);
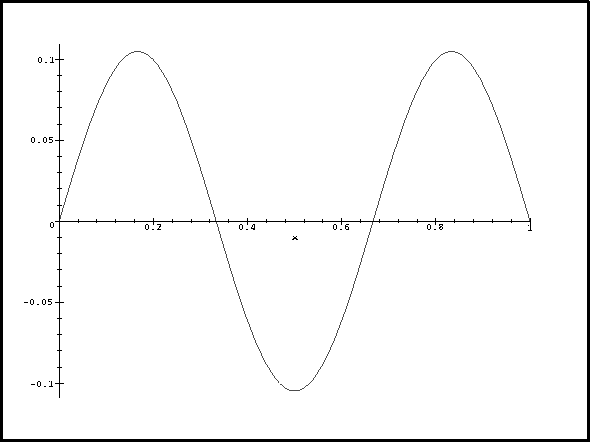
Now let's think about this. For n=1, the probability density reaches a maximum in the middle where the perturbation is negative and large. This contributes a large negative term to the energy correction so deltaE1 is negative. For n=3, the probability density and perturbation have exactly the same periodicity. The contribution from the middle lobe exactly cancels that of one of the other two so we get a positive correction. We get the greatest correction for n=2 because the probability density has a node where the perturbation is most negative and is large where the perturbation reaches a maximum.
> En := n -> n^2*h^2/(8*m*L^2);
![]()
We want the energies of the unperturbed and perturbed system. For n=1,
> evalf(En(1)); evalf(En(1)+deltaE1);
![]()
![]()
For n=2,
> evalf(En(2)); evalf(En(2)+deltaE2);
![]()
![]()
For n=3,
> evalf(En(3)); evalf(En(3)+deltaE3);
![]()
![]()
I'm going to make my plot with Maple because I want an electronic
document I can post on the web. It's far easier to do this by hand.
> with(plots):
> pa := plot([t,En(1)*m*L^2/h^2,t=0..1],0..2,0..1.2,
labels=[``,`E `],xtickmarks=0):
> pb := plot([t,En(2)*m*L^2/h^2,t=0..1],0..2,0..1.2,
xtickmarks=0):
> pc := plot([t,En(3)*m*L^2/h^2,t=0..1],0..2,0..1.2,
xtickmarks=0):
> pd := plot([t,(En(1)+deltaE1)*m*L^2/h^2,t=1..2],
0..2,0..1.2,xtickmarks=0):
> pe := plot([t,(En(2)+deltaE2)*m*L^2/h^2,t=1..2],
0..2,0..1.2,xtickmarks=0):
> pf := plot([t,(En(3)+deltaE3)*m*L^2/h^2,t=1..2],
0..2,0..1.2,xtickmarks=0):
> display(pa,pb,pc,pd,pe,pf);
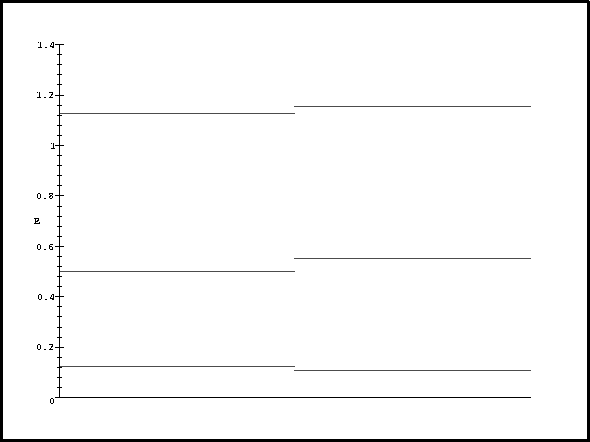
The unperturbed energy levels are on the left, the perturbed on the right.