| Excitation energy (eV) | Wavelength (nm) | Intensity |
| 16 | 262.40 | 0 |
| 226.40 | 0 | |
| 222.48 | 0 | |
| 189.34 | 0 | |
| 183.91 | 0.011 | |
| 161.56 | 0 | |
| 26 | 309.18 | 0 |
| 227.69 | 0 | |
| 223.14 | 0 | |
| 189.60 | 0 | |
| 184.23 | 0.012 | |
| 163.74 | 0 | |
| 144.17 | 0 | |
| 141.39 | 0 | |
| 62 | 340.46 | 0 |
| 232.12 | 0 | |
| 227.84 | 0 | |
| 190.35 | 0 | |
| 185.56 | 0.017 | |
| 176.08 | 0.001 | |
| 149.38 | 0 | |
| 146.73 | 0 | |
| 73 | 340.90 | 0 |
| 232.19 | 0 | |
| 227.91 | 0 | |
| 190.38 | 0 | |
| 185.59 | 0.017 | |
| 176.87 | 0.001 | |
| 149.44 | 0 | |
| 146.82 | 0 |
- (a)
- Make sure that we include all of the levels which are relatively close to the HOMO-to-LUMO gap, i.e. choose the excitation energy based on the largest gaps in the orbital energies.
- (b)
- Start with small excitations and increase the CI excitation energy by (say) 10eV at a time until no further change is observed in the spectral region of interest. In the case of fluorobenzene, an excitation energy of 60eV (or so) seems to be sufficient to give good results above 140nm.
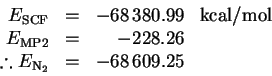
I then converted one of the nitrogen atoms to a ghost atom. The ground-state electronic configuration of a nitrogen atom is 1s22s22p3 so the multiplicity is 4. The energy of a single nitrogen atom is
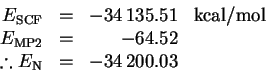
The dissociation reaction is
The experimental dissociation energy is