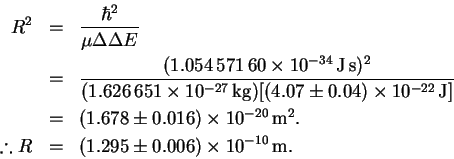I did not do an exhaustive search of the rotamers. However, for each isomer, I performed a directed search and found a relatively low energy structure:
|
| C-C: 1.51Å |
| CH3 C-H: 1.08Å | C=C: 1.32Å |
| =C-H: 1.08Å | C-C=C angle: |
|
| C-C: 1.51Å |
| CH3 C-H: 1.08Å | C=C: 1.32Å |
| =C-H: 1.08Å | C-C=C angle: |
![\begin{eqnarray*}\Delta E_J & = & E_{J+1}-E_J\\
& = & \frac{\hbar^2}{2\mu R^2}...
...+1)\left[(J+2)-J\right]\\
& = & \frac{\hbar^2}{\mu R^2}(J+1).
\end{eqnarray*}](img8.gif)
The spacing between two lines in the spectrum is therefore
![\begin{eqnarray*}\Delta\Delta E & = & \Delta E_{J+1}-\Delta E_J\\
& = & \frac{...
...R^2}\left[(J+2)-(J+1)\right]\\
& = & \frac{\hbar^2}{\mu R^2}.
\end{eqnarray*}](img9.gif)
In this case,
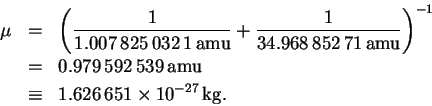
We can now solve for the bond length: