> phi := x -> exp(-beta*x^2);
> assume(beta>0); > V := x -> Vmax*(1-exp(-alpha*x^2));
> Vmax := 1e-20;
> alpha := 1e22;
> m := 1e-26;
> hbar := (6.626e-34)/(2*Pi);
As usual, we break up the variational energy into pieces:
> Kip := -hbar^2/(2*m)*int(phi(x)*diff(phi(x),x$2),
x=-infinity..infinity);
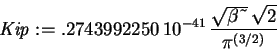
> Vip := int(phi(x)^2*V(x),x=-infinity..infinity);
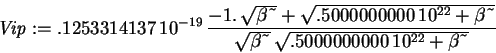
> ip := int(phi(x)^2,x=-infinity..infinity);
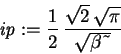
> Evar := (Kip+Vip)/ip;
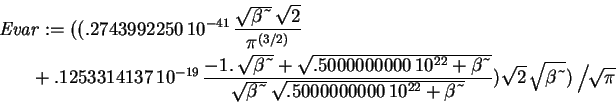
We now want to minimize this expression with respect to the variational parameter
> solve(diff(Evar,beta)=0,beta);
The positive value is the only likely one. We can verify that it minimizes the variational energy by plotting this quantity for nearby values of
> plot(Evar,beta=1e21..5e21);
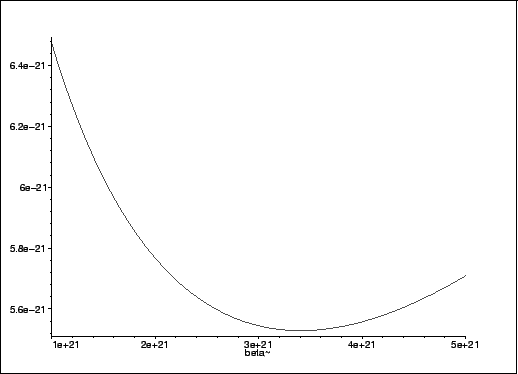
> assign(beta=.3404729218e22); > evalf(Evar);
Thus, the variational energy is