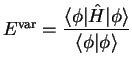Up: Back to the Chemistry 3730 test index
Chemistry 3730 Fall 2000 Quiz 3
Name:
In questions requiring the use of Maple,
outline your calculations
in standard mathematical notation. In questions using HyperChem,
give sufficient detail to allow me to reproduce your calculation.
Note that information required for
the solution of these problems is given on the last page of this paper.
- 1.
- Suppose that a particle in one dimension
experiences a potential
where
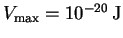 and
and
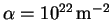 .
The particle has a mass of
.
The particle has a mass of
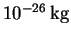 .
Compute the variational energy using the trial wavefunction
.
Compute the variational energy using the trial wavefunction
[10 marks]
Notes and hints: Maple will need to know that
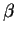 is a positive
parameter. Don't forget that eu is exp(u) in
Maple. Once you have determined the proper value of
is a positive
parameter. Don't forget that eu is exp(u) in
Maple. Once you have determined the proper value of
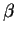 ,
you can use subs() to substitute it
into your variational energy, or assign() to
permanently assign the value.
Be sure to state the units of the energy.
,
you can use subs() to substitute it
into your variational energy, or assign() to
permanently assign the value.
Be sure to state the units of the energy.
- 2.
- There are two ways to calculate the ionization energy of an
atom or molecule:
- (a)
- Calculate the ground state energies of the
atom or molecule before and after removal of the
electron. The ionization
energy is the difference between these two energies.
- (b)
- According to Koopmans's theorem, the ionization energy
is roughly equal to the negative of the
energy of the orbital from which
the electron is to be removed.
Using the 6-31G basis set, calculate the ionization energy of
lithium using
both methods. Compare your two answers to each other and to the
experimental ionization energy (available through HyperChem's
periodic table). [10 marks]
Note: In order for this to work properly, your two ab initio
calculations should use identical computational methods.
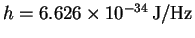
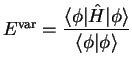
1eV = 23.06055kcal/mol



Up: Back to the Chemistry 3730 test index
Marc Roussel
2000-10-30
![]() is a positive
parameter. Don't forget that eu is exp(u) in
Maple. Once you have determined the proper value of
is a positive
parameter. Don't forget that eu is exp(u) in
Maple. Once you have determined the proper value of
![]() ,
you can use subs() to substitute it
into your variational energy, or assign() to
permanently assign the value.
Be sure to state the units of the energy.
,
you can use subs() to substitute it
into your variational energy, or assign() to
permanently assign the value.
Be sure to state the units of the energy.