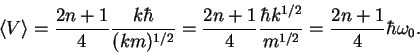> psi_pib := (n,x,L) -> sqrt(2/L)*sin(n*Pi*x/L);
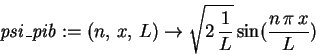
Computing probabilities is then a straightforward matter:
> evalf(int(psi_pib(1,x,L)^2,x=0..L/4));
.09084505693
> evalf(int(psi_pib(2,x,L)^2,x=0..L/4));
.2500000000
> evalf(int(psi_pib(3,x,L)^2,x=0..L/4));
.3030516477
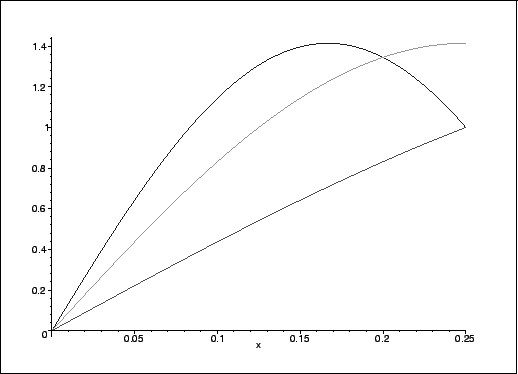
To understand why these values are as they are, we consider the shapes of the wavefunctions. It is useful to focus on the leftmost quarter for which we computed occupation probabilities:
> plot([psi_pib(1,x,1),psi_pib(2,x,1),psi_pib(3,x,1)], x=0..1/4,color=[red,green,blue]);
A := n -> sqrt(beta/(2^n*n!*sqrt(Pi)));
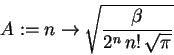
> with(orthopoly,H);
[H]
> psi := (n,x) -> A(n)*H(n,beta*x)*exp(-beta^2*x^2/2);
> assume(beta>0);We now proceed to compute the expectation values:
> int(psi(0,x)*1/2*k*x^2*psi(0,x),x=-infinity..infinity);
> int(psi(1,x)*1/2*k*x^2*psi(1,x),x=-infinity..infinity);
> int(psi(2,x)*1/2*k*x^2*psi(2,x),x=-infinity..infinity);
The pattern is reasonably obvious:
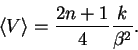
Now we use the definition of