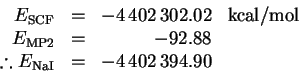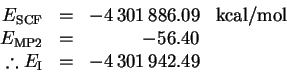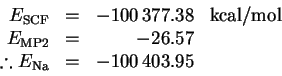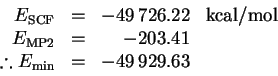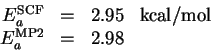Up: Back to the Chemistry 3730 assignment index
Chemistry 3730 Fall 2000 Assignment 6 Solutions
- 1.
- I first performed a UHF calculation with the STO-3G* basis set
for NaI. The SCF energy and MP2 correction are
To avoid basis-set superposition errors, we repeated the above
calculation for iodine with a sodium ghost atom:
Performing a similar calculation for the sodium atom, we get
The dissociation energy is therefore
This is a considerably better value than I obtained without
considering correlation effects (
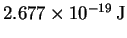 ),
although it is still quite a bit lower than the value obtained
by fitting.
),
although it is still quite a bit lower than the value obtained
by fitting.
- 2.
- (a)
-
- (b)
-
We can now calculate the activation energy by subtracting the
ground-state energy from the transition-state energy:
The activation barrier is almost exactly the same, with or
without correlation. The reason is that the correlation energy
is essentially identical in both conformations. Since the bonds
in both conformations are identical, this is fully to be
expected.
- 3.
- I started by performing a geometry optimization. I then examined
the orbital energies. There is a large gap in energy between the
core carbon orbitals and the next orbital up (orbital 7), which has an
energy of
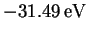 .
There is also a relatively
large gap between unoccupied
orbital 34 and orbital 35: The former has an energy of 14.27eV
while the latter is at 22.13eV. Thus, if I allow excitations
of up to 50eV in the CI calculations,
all possible combinations of excitations from the valence
orbitals to orbitals with energies under 18.5eV will be
considered. The CI calculation then has a line with nonzero
intensity at 137.55nm. Lines corresponding to lower
excitation energies all have zero intensity. Because most
UV-visible spectrometers can only record spectra down to 180nm
or so, benzene is unlikely to have a very strong UV-visible
spectrum.
.
There is also a relatively
large gap between unoccupied
orbital 34 and orbital 35: The former has an energy of 14.27eV
while the latter is at 22.13eV. Thus, if I allow excitations
of up to 50eV in the CI calculations,
all possible combinations of excitations from the valence
orbitals to orbitals with energies under 18.5eV will be
considered. The CI calculation then has a line with nonzero
intensity at 137.55nm. Lines corresponding to lower
excitation energies all have zero intensity. Because most
UV-visible spectrometers can only record spectra down to 180nm
or so, benzene is unlikely to have a very strong UV-visible
spectrum.



Up: Back to the Chemistry 3730 assignment index
Marc Roussel
2000-12-03