> Evib := n -> w0*(n+1/2) - wa*(n+1/2)^2;
where we are using the shorthand
> nvals := [0,1,2,3];
> Evals := [2.8368,8.4883,14.110,19.702];
> with(stats):
> fitted_eq :=
fit[leastsquare[[n,En],En=Evib(n),{w0,wa}]]([nvals,Evals]);
Thus we have
> hbar := 6.626069e-34/(2*Pi);
> omega0 := 5.681132253e-21/hbar;
The anharmonicity constant is
> chi := .1485366631e-1/5.681132253;
The anharmonicity constant is dimensionless.
The isotopic mass of sodium is (in amu)
> mNa := 22.98976967;
and for iodine, we have
> mI := 126.904468;
The reduced mass (in kg) is therefore
> mu := 1/(1/mNa+1/mI)/1000/6.02214199e23;
The bond stiffness is therefore
> k := evalf(omega0^2*mu);
k := 93.79792525
Thus,
> DM := 5.681132253e-21/(4*chi);
To get the dissociation energy, we need to subtract off the zero-point energy:
> De := DM - 1/2*5.681132253e-21*(1-1/2*chi);
Our spectroscopic calculation therefore gives a dissociation energy of
I saved this structure to disk, then converted the sodium atom to a ghost atom. Thus I computed the energy of an iodine atom avoiding basis set superposition error:
I loaded the optimized NaI structure back into my workspace and converted the iodine atom to a ghost atom. The following single-point calculation gave me
The energy of separated sodium and iodine atoms is therefore
The difference in energy between the molecule and separated atoms (the dissociation energy) is
The answers we get from the spectroscopic calculation and from the computational method are wildly different from each other.
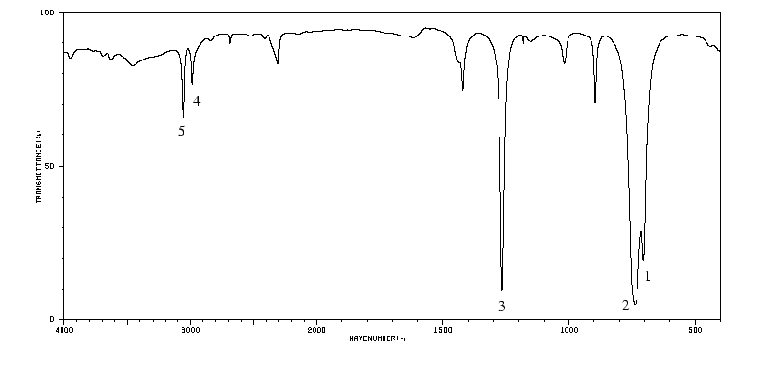
- 1
- This is a symmetric stretching mode involving mostly the C-Cl bonds.
- 2
- This is the corresponding C-Cl antisymmetric stretch.
- 3
- This normal mode is a ``wagging'' mode in which the two H atoms rock back-and-forth in concert.
- 4
- The identification of this and the next mode are much less certain. However, it seems likely that this mode is a symmetric stretch involving mostly the C-H bonds.
- 5
- If the prior identification is correct, this would be the corresponding antisymmetric stretch.