> phi := r -> exp(-alpha*r^2);
> assume(alpha>0);We break up the variational energy into three pieces, as usual:
> Kip := -hbar^2/(2*mu)*int(int(int(phi(r)*diff(r^2*diff(phi(r),r),r)
*sin(theta),r=0..infinity),theta=0..Pi),phi=0..2*Pi);
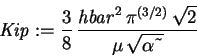
> Vip := -Z*e^2/(4*Pi*epsilon0)*int(int(int(phi(r)^2*r*sin(theta),
r=0..infinity),theta=0..Pi),phi=0..2*Pi);
> ip := int(int(int(phi(r)^2*r^2*sin(theta),r=0..infinity),
theta=0..Pi),phi=0..2*Pi);
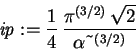
> Evar := (Kip+Vip)/ip;
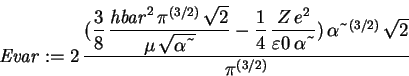
We minimize the variational energy with respect to the variational parameter
> solve(diff(Evar,alpha)=0,alpha);
> assign(alpha=1/18*Z^2*e^4*mu^2/(Pi^3*hbar^4*epsilon0^2));We can now evaluate the variational energy:
> simplify(Evar);
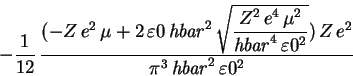
> assume(Z>0); > assume(e>0); > assume(mu>0); > assume(hbar>0); > assume(epsilon0>0); > simplify(%);
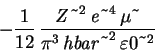
Now to compute the percent error:
> Eexact := -Z^2*e^4*mu/(32*Pi^2*epsilon0^2*hbar^2);
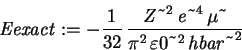
> simplify((Eexact-Evar)/Eexact);
> evalf(%);
.1511736370
Thus, the error in the Gaussian approximation is about 15%.
- (a)
- Very similar results are obtained with the
6-311G and D95* basis sets. However, the
former basis set uses 13 basis functions while the
latter uses 16. Accordingly, the 6-311G basis set is the
better one to use. With this basis set we find a
ground-state energy of
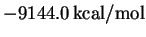 .
.
- (b)
- Adding extra basis functions doesn't reduce the energy much in this case. This suggests that the 6-311G basis set is large enough to reach the Hartree-Fock limit for beryllium.
- (a)
- I expect
Fe3+ to have a half-filled 3d
subshell so the spin multiplicity I used was 6.
I performed an STO-6G* calculation, adding an spd
shell with an orbital exponent of 21. The computed
energy was
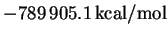 .
.
- (b)
- The computed electronic configuration is
1s22s22p63s23p63d5.This agrees with what we would have predicted based on the usual rules.