Use the variational method to find an approximation to the ground-state energy of a hydrogenic atom with this Gaussian wavefunction. What is the percent error? [10 marks]
Hints and notes: This problem can be solved analytically.
Maple will need to know that ![]() is positive.
Remember that, if you later want to give
is positive.
Remember that, if you later want to give ![]() a value, you
will have to use assign(). You may find at some point
that you want Maple to simplify an expression involving a square
root. If so, Maple will also need to know that the quantities
under the square root are positive.
In Maple, eu is exp(u) and
a value, you
will have to use assign(). You may find at some point
that you want Maple to simplify an expression involving a square
root. If so, Maple will also need to know that the quantities
under the square root are positive.
In Maple, eu is exp(u) and ![]() is Pi.
is Pi.
The volume element in spherical polar coordinates is
The angles have the following ranges:
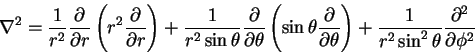
but if you think about it, you will realize that you don't need all these terms in this problem. The energy of a hydrogenic atom is given by
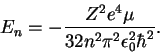
- (a)
- Find the best basis set with which to compute the
atomic orbitals of beryllium. The best basis set gives
the lowest energy with the fewest basis functions.
Your answer should include
- i.
- the basis set,
- ii.
- the calculated energy, and
- iii.
- the number of basis functions and Gaussians.
- (b)
- For your best basis set, does it help to add extra basis functions? If so, give an example. [2 marks]
- (a)
- Calculate the ground-state energy of
Fe3+.
Give complete details of the calculation. Your grade on this
question will be based on
- i.
- correctness,
- ii.
- finding a reasonably low energy,
- iii.
- the use of a sensible basis set, and
- iv.
- explanations of your computational procedures which are adequate to allow me to reproduce your results.
Hints: You will need to do a UHF calculation. Make sure to use the correct spin multiplicity.
- (b)
- For your best basis set, what is the computed electronic
configuration? Does this agree with your expectations
for this ion? [3 marks]
Hint: You can get this information from the orbital window of HyperChem, but since you did a UHF calculation, you have to put the two sides of the orbital diagram together to get the configuration.