![]()
Start by calculating the equilibrium constant:
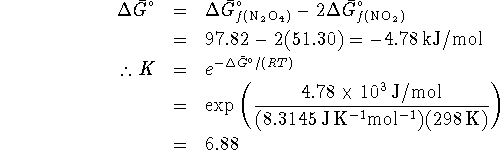
The equilibrium constant is related to the equilibrium
activities by ![]() . In a
rigid container at constant temperature,
. In a
rigid container at constant temperature, ![]() . It
follows (by stoichiometry) that
. It
follows (by stoichiometry) that ![]() . We therefore get the quadratic equation
. We therefore get the quadratic equation
![]()
or
![]()
This equation has two solutions:
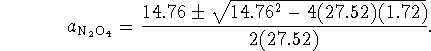
One solution ( ![]() ) leads to a negative activity for the nitrogen dioxide
and is therefore physically inadmissible.
The other solution is
) leads to a negative activity for the nitrogen dioxide
and is therefore physically inadmissible.
The other solution is ![]() which gives
which gives
![]() . Thus the equilibrium ratio of
. Thus the equilibrium ratio of
![]() to
to ![]() is 0.92.
is 0.92.
![]()
![]() at the boiling point. At
the normal boiling point,
at the boiling point. At
the normal boiling point, ![]() .
Thus we have
.
Thus we have
![]()
or
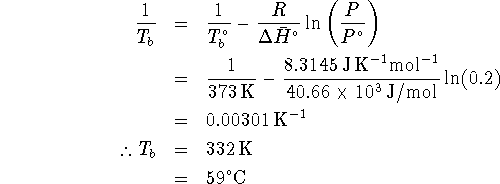
This temperature is sufficiently high that liquid water could still exist on this planet.
- We can read this off directly from the differential of
A:

- We start with the answer to question 3a:
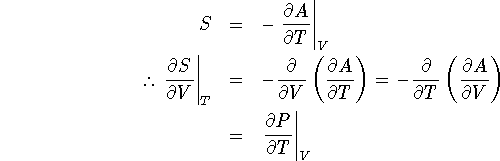
- For an ideal gas, P = nRT/V so

![]()
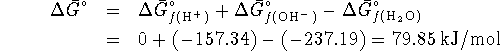
The equilibrium constant at 298K is therefore
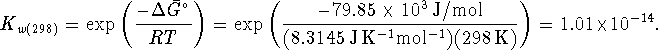
Therefore
![]()
at 298K, giving us a ![]() of 10.1.
of 10.1.
To get the ![]() , we need to know the equilibrium
constant at this temperature. For this we need
, we need to know the equilibrium
constant at this temperature. For this we need
![]() .
.
- Two reactions are coupled if they can be made to occur
together in such a way that one reaction drives the
other. This requires that the overall free energy
change for the two reactions taken together be negative.
For example, fructose-6-phosphate is made from fructose
by reaction with phosphate:
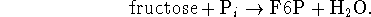
This reaction can be made to yield more product if it is coupled to a reaction with a very negative free energy change which produces phosphate such as ATP hydrolysis:
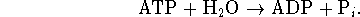
The overall process is
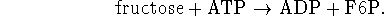
- Le Chatelier's principle states that a chemical system will react in such a way as to partially offset an applied stress. For example, increasing the temperature shifts the equilibrium of exothermic reactions toward the reactants because exothermic reactions absorb heat when reactants are formed from the products. Coupled reactions also rely on Le Chatelier's principle: In the example above, we might say that production of fructose-6-phosphate is increased because ATP hydrolysis supplies one of the reactants of the other reaction. The system responds by reacting away some of this phosphate.
![]()
Maximum transport (requiring the minimum ratio of ATP to ADP) is achieved
when ![]() , i.e. when
, i.e. when
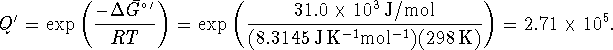
However,
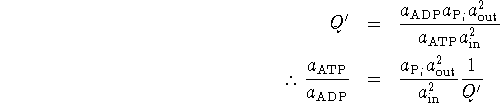
In the biochemists' standard state, ![]() ,
, ![]() and
and ![]() .
Therefore
.
Therefore
![]()