Next: Answer one of the
Up: Chemistry 2720 Spring 1998
Previous: Chemistry 2720 Spring 1998
Value of this section: 80 marks
- Calculate the equilibrium constant for the reaction
of zinc (II) oxide with fluorine to form
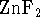 and
oxygen at 298K.
[5 marks]
and
oxygen at 298K.
[5 marks] - A university student decides to go on an all-sugar diet.
Sugar delivers 4.0kcal/g of metabolizable energy. During the
first month, the student gains 4kg, stored as fat. The
metabolizable energy equivalent of fat is 9.5kcal/g. By how
much should the student cut back his daily intake of sugar to
stop gaining weight? [5 marks]
- A thermodynamicist finds that the enthalpy of formation of
ozone (
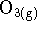 ) at
) at 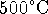 is
140kJ/mol. What is the value of the enthalpy of formation at
298K? [8 marks]
is
140kJ/mol. What is the value of the enthalpy of formation at
298K? [8 marks] - What is the boiling point of a solution made by dissolving
3g of eugenol (oil of cloves, molar mass 164.20g/mol) in
300g of benzene? The enthalpy of vaporization of benzene is
34.7kJ/mol at its boiling point of 353.3K and the molar mass
of benzene is 78.11g/mol. [10 marks]
- 1.05g of a newly isolated protein is dissolved in 500mL of
water at 298K. The osmotic pressure of this solution is 260Pa.
What is the molar mass of the protein? [6 marks]
- The normal boiling point of bromine is 332.35K and its
enthalpy of vaporization at this temperature is 29.45kJ/mol.
Calculate the boiling point of bromine at 0.89atm,
the normal atmospheric pressure in Lethbridge.
[6 marks]
- The chemiosmotic theory of ATP synthesis suggests that
mitochondria use the oxidation of
carbohydrates and fats to operate a proton pump resulting in an
internal pH of 7.0 when the pH of the surrounding cytoplasm is 5.5.
When these protons spontaneously flow back in across the
mitochondrial membrane, their free energy is harnessed to
synthesize ATP. The concentrations of ATP, ADP and phosphates
in the mitochondria are 1, 1 and 2.5mM, respectively. For the
ATP synthesis reaction
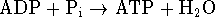 ,
,
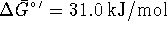 at 298K.
at 298K.
- How much free energy is required to synthesize 1mol
of ATP in a mitochondrion at 298K? [4 marks]
- How much free energy is produced by moving 1mol of
protons into the mitochondrion from the outside?
[4 marks]
- What is the minimum number of protons which must cross
the membrane to
synthesize one molecule of ATP? [2 marks]
Note: Report the smallest sufficiently large whole number.
- A coal-fired power plant is designed to produce 12MW of
electricity. (This is a small unit that might be used to power
a pulp-and-paper mill or other relatively energy-intensive
factory.) The coal is used to heat steam to a temperature of
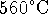 . After running through the turbines, the
steam has been condensed and the liquid water
cooled to
. After running through the turbines, the
steam has been condensed and the liquid water
cooled to 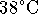 .
.
- Calculate the minimum amount of heat which must be
generated to produce
the desired amount of electricity. [5 marks]
- Calculate the amount of heat required to bring 1kg of
liquid water from
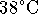 up to steam at
up to steam at 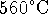 . [6 marks]
. [6 marks] - How fast must water be circulated through the heating
unit to generate the
required amount of electricity? [2 marks]
- The enthalpy of combustion of coal is 29MJ/kg.
What is the minimum rate at which coal must be burned
to sustain the required level of power production?
[2 marks]
- Acetic acid (
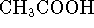 ) has an acid dissociation constant of
) has an acid dissociation constant of 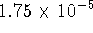 at 298K.
at 298K.
- Calculate the pH of a 0.1mol/L solution
of acetic acid in water. [5 marks]
- Calculate the concentration of acetate ions in this
solution. Do a full Debye-Hückel calculation.
[10 marks]
Hints: Treat the acetic acid as ideally behaving.
The method is analogous to that used to
compute solubilities.
Next: Answer one of the
Up: Chemistry 2720 Spring 1998
Previous: Chemistry 2720 Spring 1998
Marc Roussel
Thu Apr 23 12:12:43 MDT 1998
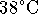 up to steam at
up to steam at 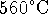 . [6 marks]
. [6 marks]