- First, we decode the question. Reversible means
that
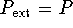 .
Isothermal means that the
temperature is constant.
The work is
.
Isothermal means that the
temperature is constant.
The work is
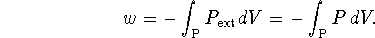
According to the van der Waals equation,
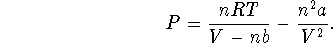
Therefore
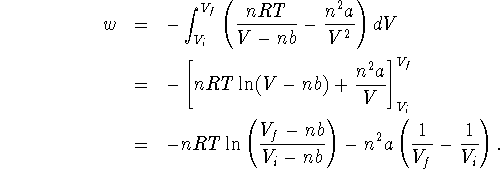
- To answer this question, we simply need to substitute in
the values (carefully). It is essential in this kind of
calculation to use SI units throughout. In this case,
all of the data are given in SI units so that doesn't
take a lot of thinking. Given that all of the data are
in SI units, the answer will come out in SI units.
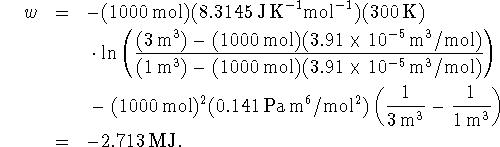
-
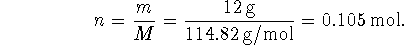
We also have to convert the temperatures from degrees Celsius to Kelvin because the heat capacity formula requires T in these units.
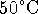 is 323K and
is 323K and
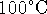 is 373K. I won't write down the
units in the following calculation because it's highly
tedious. I'm being careful to use SI units everywhere,
so the answer should come out in SI units.
is 373K. I won't write down the
units in the following calculation because it's highly
tedious. I'm being careful to use SI units everywhere,
so the answer should come out in SI units.
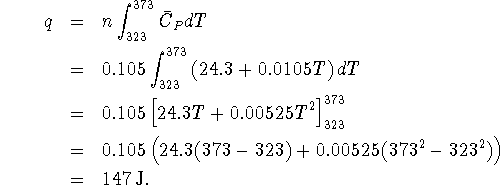
- If the heat capacity is taken to be a constant, then we
simply have
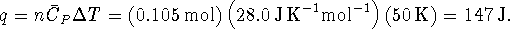
The error is negligible.
![]()
At constant pressure, the heat is just the enthalpy change. The enthalpy change for this process is
![]()
using data from the table in the assignment. The reaction is endothermic, i.e. it absorbs heat, so the water will drop in temperature.
Since the reaction is taking place in an insulated (adiabatic) container, no heat is either gained or lost from the water/KBr system. Therefore
![]()
where ![]() is the heat of reaction (
is the heat of reaction ( ![]() ) and
) and ![]() is the heat gained or lost by the
water (
is the heat gained or lost by the
water ( ![]() ). Therefore
). Therefore
![]()
or
![]()
We have most of the required data, except for
![]()
We can now calculate the temperature change:
![]()
Since a Kelvin is of exactly the same size as a degree Celsius,
and since the initial temperature was ![]() , the
final temperature is
, the
final temperature is ![]() .
.
![]()
of lead.
The lead starts out well below its melting point, at a
temperature of ![]() or 308K. The heat
required to raise the lead to its melting point is therefore
or 308K. The heat
required to raise the lead to its melting point is therefore
![]()
The heat required to melt the lead is
![]()
Thus, every hour, the furnace must supply ![]() of heat. This is a rate of power consumption
of
of heat. This is a rate of power consumption
of
![]()