We want to calculate the work along a reversible path ( ![]() ) so
) so
![]()
Since the gas is ideal, we can replace P by nRT/V. Furthermore, T = kV along the path to which the process is constrained, so P = nRk. Thus
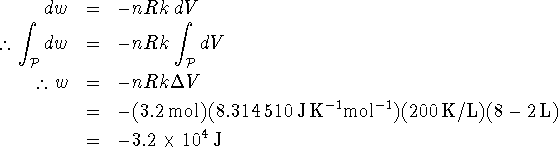
Note that it was not necessary to convert the volumes to ![]() since the constant k was given in K/L.
since the constant k was given in K/L.