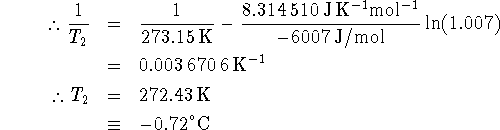- If the reaction is exothermic, reduce the temperature. If the reaction is endothermic, increase the temperature.
- Use coupled reactions: Add reactants which combine to
form either
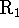 or
or 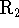 such that
the free energy change for
the overall reaction is more negative than the free
energy change for the original reaction.
such that
the free energy change for
the overall reaction is more negative than the free
energy change for the original reaction.
- Possible: n=8,
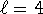 ,
, 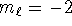
-
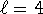 and n=4 but
and n=4 but 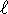 should be less than n
so this is not a possible hydrogenic state.
should be less than n
so this is not a possible hydrogenic state. -
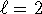 and
and 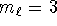 but
but 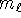 can't be larger
than
can't be larger
than 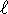 so this is not a possible hydrogenic state.
so this is not a possible hydrogenic state.
- The reaction is
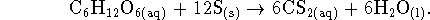
For this reaction,
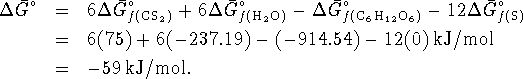
To calculate the maximum work, we need
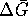 :
:
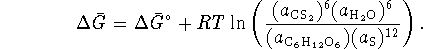
The activity of the solvent (water) is normally taken to be 1 and the activity of solid sulfur is exactly 1.
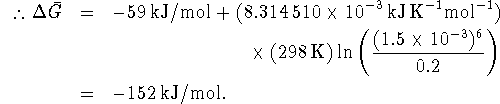
A cell could therefore extract up to 152kJ/mol of work from the oxidation of glucose by sulfur under the stated conditions.
-
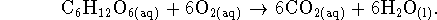
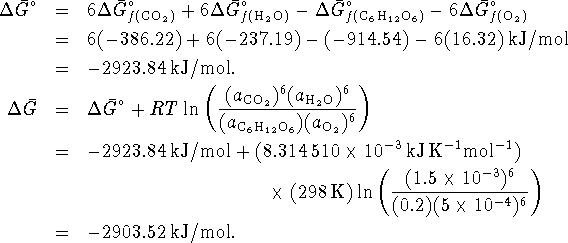
A cell could therefore extract up to 2903.52kJ/mol of work from the oxidation of glucose by oxygen under the stated conditions, which is much more than is obtained from oxidation by sulfur under similar conditions. There is therefore a huge metabolic advantage to using oxygen rather than sulfur as an oxidizing agent.
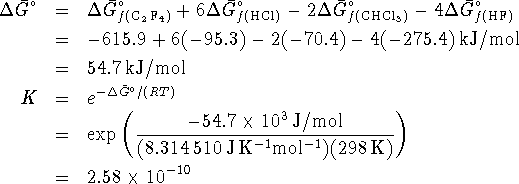
This equilibrium ratio is extremely small and suggests that very
little of the reactants will in fact be consumed.
Accordingly, we guess that the final pressures of
![]() and HF will be close to the initial pressures.
By stoichiometry,
and HF will be close to the initial pressures.
By stoichiometry, ![]() .
Our equilibrium condition then reads
.
Our equilibrium condition then reads
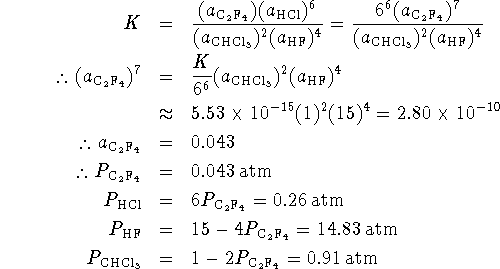
Note: The exact answer is ![]() ,
, ![]() ,
,
![]() ,
, ![]() so our approximation gives pretty good
results.
so our approximation gives pretty good
results.
![]()
For the process ![]() , the equilibrium constant is
, the equilibrium constant is
![]() where X is the mole fraction
of the liquid. We will take
where X is the mole fraction
of the liquid. We will take ![]() to be the normal freezing
point of pure water (273.15K, X=1) while
to be the normal freezing
point of pure water (273.15K, X=1) while ![]() will be the
freezing point of the solution. For the water in the solution,
will be the
freezing point of the solution. For the water in the solution,
![]()
The equilibrium constant for the solution at its freezing point is therefore K=1/0.993=1.007.