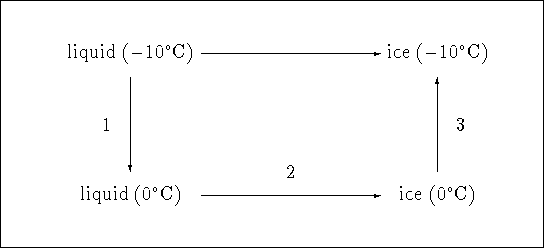
Consider the following diagram:
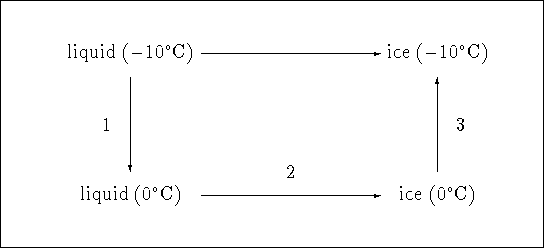
The path consisting of steps 1, 2 and 3 produces the same overall change
as the freezing of ice at ![]() . All we need to do is
to calculate
. All we need to do is
to calculate ![]() for each step and then add them up:
for each step and then add them up:
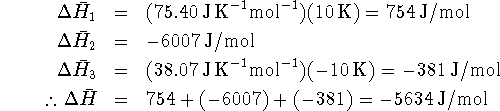
This is significantly different from the enthalpy change at
![]() . Fortunately, for chemical reactions (but not for
phase changes, as this example shows), the variation of
enthalpy change with temperature is much less than this because the difference
in heat capacities between reactants and products is not normally as
large as it is for ice and water. Accordingly, we can usually behave as
if the enthalpy changes were constant.
. Fortunately, for chemical reactions (but not for
phase changes, as this example shows), the variation of
enthalpy change with temperature is much less than this because the difference
in heat capacities between reactants and products is not normally as
large as it is for ice and water. Accordingly, we can usually behave as
if the enthalpy changes were constant.