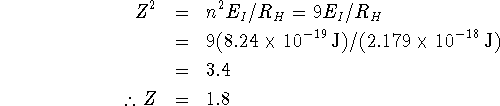
The valence electron in sodium is in a 3s orbital so n=3. Therefore
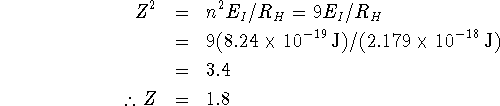
Sodium has eleven electrons. Ten of these occupy the noble-gas core orbitals so we would think that the effective nuclear charge experienced by the valence electron would be about 1 (11-10). It is a little higher than this. This indicates that the core electrons do not perfectly screen the valence electron. This makes sense given that the 3s orbital has significant probability density near the nucleus. At least that fraction of the probability density cannot be screened by the other electrons.