Quiz 1 Name(s):
Calculate the work done (per mole) when water is compressed isothermally (at 298K) and reversibly from 1 to 300atm of pressure. The following simplified form of the equation of state of water gives the molar volume as a function of the pressure:
![]()
where ![]() ,
, ![]() and P is in Pascals
(1atm = 101325Pa).
and P is in Pascals
(1atm = 101325Pa).
Reminder: 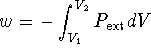 .
.
Hint 1: It's a lot easier to solve this problem if you don't try to use numerical values until the end.
Hint 2: If you work consistently in SI units (Pa,
![]() ), the answer will come out in SI units, in this case in J/mol.
), the answer will come out in SI units, in this case in J/mol.