![]()
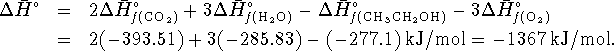
![]() .
Treating all gases as ideal, we can replace
.
Treating all gases as ideal, we can replace ![]() by
by ![]() , where
, where ![]() is the change in the
number of stoichiometric equivalents of gas. In this case,
is the change in the
number of stoichiometric equivalents of gas. In this case,
![]() . Therefore
. Therefore
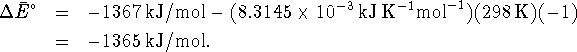
- The volume of a breath can be used with the temperature
and pressure to determine the number of moles of gas in
each breath. In order to get the right answer, it is
necessary to convert all measurements to SI units:
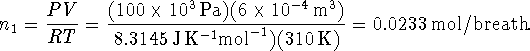
By multiplying this number by the number of breaths per minute and then by the number of minutes in an hour and by the number of hours of activity, we get the total number of moles of air which pass into the lungs:
The air is warmed by
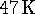 from
from 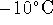 to
to
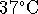 .
The heat required to heat the air is therefore
.
The heat required to heat the air is therefore
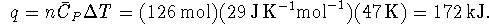
- We need the answer of the last question
and the data of this question to be in compatible units.
We might for instance convert the heat needed to warm
the air into kcal:
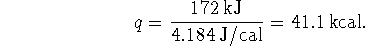
The mass of blubber which the hunter must eat to offset the energy loss from this source is therefore
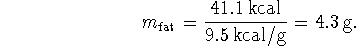
- A process is spontaneous if
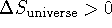 . We split the change of entropy of the universe into
two parts, one for the system (ice) and one for the
surroundings (room at
. We split the change of entropy of the universe into
two parts, one for the system (ice) and one for the
surroundings (room at 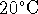 ).
Since melting is an isothermal process and the
surroundings are assumed to have sufficient heat
capacity that their temperature is not affected by the
melting of the ice, both calculations are trivial:
).
Since melting is an isothermal process and the
surroundings are assumed to have sufficient heat
capacity that their temperature is not affected by the
melting of the ice, both calculations are trivial:
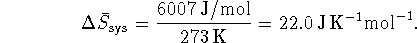
(Note that these calculations and those of the next section can also be performed on a mass basis rather than on a molar basis.) The heat that goes into melting the ice is drawn from the surroundings:
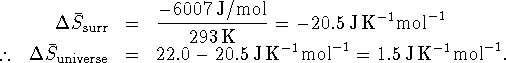
Since
 , ice will melt
in a room at
, ice will melt
in a room at 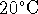 .
. - If
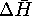 and
and 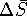 are roughly
independent of temperature, we can use
are roughly
independent of temperature, we can use 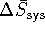 as calculated in the previous part of this problem.
as calculated in the previous part of this problem.
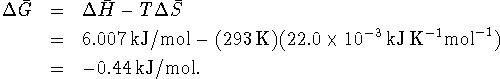
Since
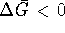 , the process is spontaneous.
, the process is spontaneous.

The maximum work available from the hydrolysis reaction is therefore 34.4kJ/mol.
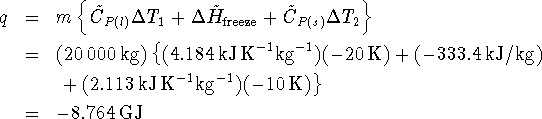
The coefficient of performance of the freezer is
![]()
The work required to freeze these turkeys is therefore
![]()