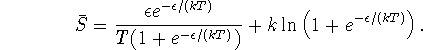![]()
If we let the energy of the ground state ( ![]() )
be 0 (which we can do
since we are free to set the zero of energy anywhere we want)
then
)
be 0 (which we can do
since we are free to set the zero of energy anywhere we want)
then
![]()
The partition function is
![]()
so the entropy becomes
![]()
- Again, let the energy of the ground state be zero.
(This is not necessary but considerably simplifies the
calculations.)
The probability of occupation of level i is
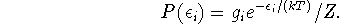
For the ground state, we get P(0)=1/Z while for the excited state,
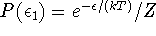 because the level is nondegenerate.
Let us say that the probability of occupation of level 1
is significant when the ratio
because the level is nondegenerate.
Let us say that the probability of occupation of level 1
is significant when the ratio
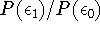 becomes greater than 0.01.
(This is arbitrary and we could have picked a different
value. The result would have been quantitatively
different, but not qualitatively different.)
Thus we want to know at what temperature
becomes greater than 0.01.
(This is arbitrary and we could have picked a different
value. The result would have been quantitatively
different, but not qualitatively different.)
Thus we want to know at what temperature
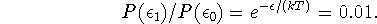
By taking a natural logarithm of both sides of this equation and rearranging, we get
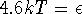 or
or

This kind of calculation shows that an energy level only contributes significantly to the thermodynamic properties of a substance if its energy is comparable to kT.
- The partition function is

The internal energy is therefore
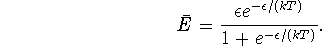
Thus the entropy is