- Suppose that gaseous methanol is burned at constant pressure in an internal combustion engine with upper and lower operating temperatures of 400 and 900K, respectively. One of the products is water vapour. How much work can be obtained per mole of methanol under these conditions?
- Suppose that liquid methanol is instead oxidized in a fuel cell. The methanol is kept separate from all other reactants and products at all times in the particular cell used. The carbon dioxide produced shows up in a gaseous stream flowing through another part of the cell at a pressure of approximately 0.1atm. Air also flows through so that the oxygen pressure is always 0.22atm. How much work can be obtained from this fuel cell at 298K?
- Calculate the voltage generated by the fuel cell
under the conditions outlined above.
Hint: The oxygen half-reaction is
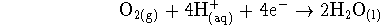
![]()
The zinc and copper solutions are both produced by dissolving the appropriate sulfates. The concentration of zinc sulfate in the left half-cell is 0.005mol/L and the concentration of copper sulfate in the right half-cell is 0.002mol/L. Do a full Debye-Hückel calculation to compute the electrochemical potential of this cell at 298K. Is Debye-Hückel theory in fact applicable to this problem?
